Abstract
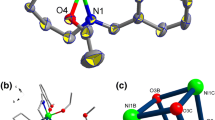
The structures of the title compounds, 1,2,4,7-tetra(carboxymethyl)cubane 1 and 1,2,3,5,7-penta(carboxymethyl)cubane2, have been determined.1 crystallized in the space group\(P\bar 1\) with cell dimensionsa=6.098(2),b=10.686(2),c=13.459(2) Å, α=69.82(1), β=77.01(1), γ=74.00(1)°, while2 crystallized in the monoclinic space group P21/c with cell dimensionsa=15.139(2),b=12.775(1),c=9.849(2) Å, β=107.01(1)°. These molecules were derived from their parent carboxylic acids by esterification with methanol. They are unusual for several reasons. The first is that they both contain substituents on adjacent carbon atoms in the cubane framework. There are only a few reports in the literature of this type of cubane structure. The second molecule is unique in that it has five substituents attached to a cubane moiety. This is the one of the few cubane derivatives with more than four substituents to be structurally characterized and results in a cubane molecule in which there are three faces with three substituents and three faces with only two substituents.
Similar content being viewed by others
References
Eaton, P. E.Angew. Chem. Int. Ed. Engl. 1992,31, 1421.
Bashir-Hashemi, A.,Angew. Chem. Int. Ed. Engl. 1993,32, 612.
Eaton, P. E.; Pramod, K.; Gilardi, R.J. Org. Chem 1990,55, 5746.
Bashir-Hashemi, A.; Ammon, H. L.; Choi, C. S.J. Org. Chem. 1990,55, 416.
Ammon, H. L.; Bashir-Hashemi, A.,Acta Cryst. C. (Cr. Str. Comm.) 1993,49, 1641.
Eaton, P. E.; Xiong, Y.; Gilardi, R.J. Am. Chem. Soc. 1993,115, 10195.
Gilardi, R.; George, G.; Karle J.; Eaton, P. E.; Rao, M.J. Heterocyclic Chem. 1993,30, 1389.
See ref 2..
Sheldrick, G. M.,1986.SHELXTL, Crystallographic System; Siemens Analytical Instrument Division: Madison, Wis.
Cromer, D. T.; Waber J. T.International Tables for X-Ray Crystallography Vol. IV; The Kynoch Press, Birmingham England; 1974.
Cromer, D. T.International Tables for X-Ray Crystallography, Vol IV; The Kynoch Press: Birmingham, England, 1974.
Sheldrick, G. M.; Schneider, T. R.;Methods in Enz, in press. This is a full-matrix least-squares refinement package that uses all data and refines on F2 rather than the traditional F. The various parameters used in this refinement process are defined as follows:\(\begin{gathered} R_1 = \Sigma |F_o - F_c |/\Sigma F_o \hfill \\ wR_2 = \{ \Sigma [w(F_o ^2 - F_c ^2 )^2 ]/[w(F_o ^2 )^2 ]\} ^{1/2} , \hfill \\ \end{gathered}\) wherew=1/[σ2(F o)+(aP)2+bP] anda andb are variable parameters whose optimal values are usually suggested by the program during the refinement process. The goodness-of-fit parameter (S) is based onF 2 and defined as:\(s = \{ \Sigma [w(F_o ^2 - F_c ^2 )^2 ]/[n - p]\} ^{1/2} ,\) wheren is the number of reflections andp is the total number of parameters refined.
Since the tetrasubstituted molecule has inversion symmetry only the metrical parameters for the unique part of the molecule are included in the supplementary material. Thus the numbering scheme used for this molecule in the deposited material is necessarily different from that used in table 4 where every carbon atom in the cubane skeleton has a unique label.
Allen, F. H.Acta Cryst. 1980,B36, 81.
Fleischer, E. B.J. Am. Chem Soc. 1964,86, 3889.
Eaton, P. E.; Shankar, B. K. R.; Price, G. D.; Pluth, J. J.; Gilbert, E. E.; Alster, J.; Sandus, O.J. Org. Chem. 1984,49, 185.
Lex, J.; Ermer O.Angew. Chem., Int. Ed. Engl. 1987,26, 447.
Ammon, H. L.; Choi, C. S.; Reddy, S.Acta Cryst. C. (Cr. Str. Comm.) 1988,44, 1671.
Gilardi, R. G.; Maggini, M.; Eaton, P. E.J. Am. Chem. Soc. 1988,110, 7232.
Moriarty, R. M.; Khosrowshahi, J. S.; Miller, R. S.; Flippen-Anderson, J.; Gilardi, R.J. Am. Chem. Soc. 1989,111, 8943.
Hassenruck, K.; Radziszewski, J. G.; Balaji, G.; Murthy, G. S.; McKinley, A. J.; David, D. E.; Lynch, V. M.; Martin, H.-D.; Michl, J.J. Am. Chem. Soc. 1990,112, 873.
Hassenruck, K.; Murthy, G. S.; Lynch, V. M.; Michl, J.J. Org. Chem. 1990,55, 1013.
Kawai, N. T.; Gilson, D. F. R.; Britten, J. F.; Butler, I. S.; Farrell, P. G.Can. J. Chem. 1992,70, 910.
Moriarty, R. M.; Rao, M. S. C.; Tuladhar, S. M.; D'Silva, C.; Williams, G.; Gilardi, R.J. Am. Chem. Soc. 1993,115, 1194.
Ammon, H. L.; Choi, C. S.; Damvarapu, R. S.; Iyer, S.; Alster, J.Acta Cryst. C. (Cr. Str. Comm.) 1990,46, 295.
The structures of pentanitrocubane and hexanitrocubane have recently been determined in this laboratory (presented at the 14th Annual Working Group Institute on the Synthesis of High Energy Density Materials, June 8, 1995). A manuscript is in preparation reporting the full details of these structures: P. Eaton, R. Gilardi, J. Li, and K. Lukin, to be published.
This structure was determined in 1964 and is based on only 97 independent structure amplitudes. Since this is an important benchmark molecule in cubane chemistry, it seems important that this structure should be repeated for a more accurate result.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Butcher, R.J., Bashir-Hashemi, A. & Gilardi, R.D. The crystal and molecular structures of highly substituted cubanes: 1,2,4,7-tetra(carboxymethyl)cubane and 1,2,3,5,7-penta(carboxymethyl)cubane. J Chem Crystallogr 25, 661–670 (1995). https://doi.org/10.1007/BF01665973
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01665973