Summary
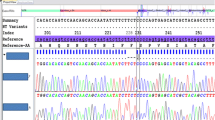
Among 20 individuals with severeα 1 antitrypsin (α1AT) deficiency we observed extremely variable clinical phenotypes ranging from rapidly progressive lung disease fatal at the age of 42 years to an asymptomatic individual with normal lung function at the age of 50 years. Eighteen subjects, including the asymptomatic one, carried the deficient Pi ZZ phenotype as determined by isoelectric focusing (IEF). Their meanα1AT serum level was 36.7±7.7 mg/dl. DNA restriction analysis showed that all of them had the classical Pi Z-allele-associated DNA haplotype, thus confirming the IEF data. Obviously not all Pi ZZ individuals will have clinical sequelae caused by this genotype. The important differences in clinical course observed could not be explained by smoking habits alone. Probably additional factors are pertinent to the pathogenesis of the lung disease associated withα1AT deficiency (defects in other genes, environmental influences other than smoking). In two patients with very lowα1AT serum levels definitive phenotyping by IEF was not possible. Therefore we investigated the molecular basis of their deficiency using polymerase chain reaction (PCR) amplification of the coding exons of theirα1AT genes and direct sequencing of the amplification products. Sequence data analysis showed that one of these patients, who had initially been phenotyped as Pi ZZ by IEF, had in fact the genotype Pi QObellinghamZ, thus explaining her lowα1AT serum level of 20 mg/dl. The other patient (α1AT serum level 3.7 mg/dl) exhibited the rare genotype Pi MheerlenQOgranite falls. Despite his nearly completeα1AT deficiency, he suffered from only moderately severe pulmonary disease at the age of 42 years.
Similar content being viewed by others
Abbreviations
- α1AT:
-
α 1-antitrypsin
- IEF:
-
isoelectric focusing
- Pi:
-
proteinase inhibitor phenotype
- PCR:
-
polymerase chain reaction
- COPD:
-
chronic obstructive pulmonary disease
- IGV:
-
intrathoracic gas volume
- Raw :
-
airway resistance
References
Barr AE, Cole DA, Clagne AE (1989) The effect of an acute phase response or pregnancy on plasmaα1-antitrypsin concentrations in persons with various S and Z phenotypes. Ann Clin Biochem 25:255–258
Cox DW (1975) A new deficiency allele ofα1-antitrypsin: Pi Mmalton. In: Peters H (ed) Protides of the biological fluids. Pergamon Press, Oxford, pp 375–378
Cox DW, Woo SLC, Mansfield T (1985) DNA restriction fragments associated withα1-antitrypsin indicate a single origin for deficiency allele Pi Z. Nature 316:79–81
Cox DW (1988) Emphysema of early onset associated with a complete deficiency ofα1-antitrypsin (Null homozygotes). Am Rev Resp Dis 137:371–375
Curiel D, Brantly M, Curiel E, Stier L, Crystal RG (1989)α1-antitrypsin deficiency caused by theα1-antitrypsin Nullmattawa gene. J Clin Invest 83:1144–1152
Faber J-P, Weidinger S, Olek K (1990) Sequence data of the rare deficientα1-antitrypsin variant Pi Zaugsburg. Am J Hum Genet (in press)
Garver RI, Mornex J-F, Nukiwa T et al. (1986)α1-antitryp-sin deficiency and emphysema caused by homozygous inheritance of non-expressingα1-antitrypsin genes. N Engl J Med 314:762–766
Graham A, Kalsheker NA, Newton CR, Bamforth FJ, Powell SJ, Markham AF (1989) Molecular characterization of threeα1-antitrypsin deficiency variants: proteinase inhibitor (Pi) Nullcardiff (Asp256 to Val); Pi Mmalton (Phe51 to deletion) and Pi I (Arg39 to Cys). Hum Genet 84:55–58
Hofker MH, Nukiwa T, van Paassen HMB, Nelen M, Kramps JA, Klasen EC, Frants RR, Crystal RG (1989) A Pro —Leu substitution in codon 369 of theα1-antitrypsin deficiency variant Pi Mheerlen. Hum Genet 81:264–268
Janus ED, Phillips NT, Carrell RW (1985) Smoking, lung function andα1-antitrypsin deficiency. Lancet I:152–154
Lieberman J, Gaidulis L, Klotz SD (1976) A new deficient variant ofα1-antitrypsin (Mduarte). Inability to detect the heterozygous state byα1-antitrypsin phenotyping. Am Rev Resp Dis 114:31–36
Meisen C, Higuchi M, Bräutigam S, Driesel AJ, Blandfort M, Olek K (1988) Prenatal diagnosis ofα1-antitrypsin deficiency using oligonucleotide probe analysis. Hum Genet 79:190–192
Muensch H, Gaidulis L, Kueppers F, So SY, Escano G, Kidd VJ, Woo SLC (1986) Complete absence of serum alantitrypsin in conjunction with an apparently normal gene structure. Am J Hum Genet 38:898–907
Nukiwa T, Satoh K, Brantly ML, Ogushi F, Fells GA, Courtney M, Crystal RG (1986) Identification of a second mutation in the protein-coding sequence of the Z typeα1antitrypsin gene. J Biol Chem 261:15989–15994
Nukiwa T, Takahashi H, Brantly M, Courtney M, Crystal RG (1987)α1-antitrypsin Nullgranite falls, a non expressingα1-antitrypsin gene associated with a frameshift to stop mutation in a coding exon. J Biol Chem 262:11999–12004
Poller W, Meisen C, Olek K (1990) DNA polymorphisms of theα1-antitrypsin gene region in patients with chronic obstructive pulmonary disease. Eur J Clin Invest 20:1–7
Saiki R, Gelfand D, Stoffel S, Scharf S, Higuchi R, Horn G, Mullis K, Erlich H (1988) Primer directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491
Satoh K, Nukiwa T, Brantly M, Garver RI, Hofker M, Courtney M, Crystal RG (1988) Emphysema associated with complete absence ofα1-antitrypsin caused by a stop codon in anα1-antitrypsin coding exon. Am J Hum Genet 42:77–83
Sifers RN, Brashears-Macatee S, Kidd VJ, Muensch H, Woo SLC (1988) A frameshift mutation results in a truncatedα1-antitrypsin that is retained within the rough endoplasmic reticulum. J Biol Chem 263:7330–7335
Takahashi H, Nukiwa T, Satoh K, Ogushi F, Brantly M, Fells G, Stier L, Courtney M, Crystal RG (1988) Characterization of the gene and protein of theα1-antitrypsin “deficiency” allele Mprocida. J Biol Chem 263:15528–15534
Wewers MD, Casolaro MA, Crystal RG (1987a) Comparision ofα1-antitrypsin levels and anti-neutrophil elastase capacity of blood and lung in a patient with theα1-antitrypsin phenotype Null-Null before and afterα1-antitrypsin augmentation therapy. Am Rev Resp Dis 135:539–543
Wewers MD, Casolaro MA, Sellers S et al. (1987b) Replacement therapy forα1-antitrypsin deficiency associated with emphysema. N Engl J Med 316:1055–1062
Whitehouse DB, Lovegrove JU, Hopkinson DA (1989) Variation inα1-antitrypsin phenotypes associated with penicillamine therapy. Clin Chim Acta 179:109–116
Wong C, Dowling CE, Saiki RK, Higuchi RG, Erlich HA, Kazazian HH (1987) Characterization of β-thalassemia mutations using direct genomic sequencing of amplified single copy DNA. Nature 330:384
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Poller, W., Faber, J.P. & Olek, K. Highly variable clinical course in severeα 1-antitrypsin deficiency — Use of polymerase chain reaction for the detection of rare deficiency alleles. Klin Wochenschr 68, 857–863 (1990). https://doi.org/10.1007/BF01662782
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01662782