Summary
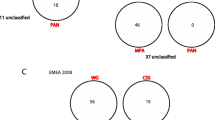
The antigenic specificity and clinical distribution of the antineutrophil cytoplasmic antibodies (ANCA) in kidney diseases have recently been extensively studied. In patients with systemic vasculitis, the great predominance of two major ANCA antigens, proteinase 3 (PR3) and myeloperoxidase (MPO), is now established. PR3 and MPO are colocalized in the azurophilic granules of neutrophils and translocated to the cell surface during activation, and thus are able to interact with autoantibodies after neutrophil preactivation. Furthermore, by comparison of amino acid and DNA sequences, it has been shown that PR3 is identical to myeloblastin, which has been described independently and is involved in the control of growth and differentiation of leukemic cells. Aside from the two major ANCA antigens, a number of neutrophil cytoplasmic antigens recognized by ANCA have been identified, including human leukocyte elastase, lactoferrin, CAP57, and cathepsin G. These rare ANCA specificities occur in a limited number of patients. The variety of ANCA antigen specificities contrasts, however, with the fact that the vast majority of ANCA-positive sera are monospecific for one single ANCA antigen.
With regard to clinical distribution, ANCA have major diagnostic significance in the four conditions in which they are frequently detected: Wegener's granulomatosis (WG), Churg and Strauss Syndrome (CSS), microscopic periarteritis (MPA), and necrotic and crescentic glomerulonephritis (NCGN). However, the initial dichotomy between MPO-associated vasculitis (NCGN, MPA) and that associated with anti-PR3 antibodies (WG) appears far from absolute.
Similar content being viewed by others
Abbreviations
- ANCA:
-
antineutrophil cytoplasm antibodies
- PR3:
-
proteinase 3
- MPO:
-
myeloperoxidase
- CAP57:
-
cationic antimicrobial protein 57 kDa
- WG:
-
Wegener's granulomatosis
- CSS:
-
Churg and Strauss syndrome
- MPA:
-
microscopic periarteritis
- NCGN:
-
necrotic and crescentic glomerulonephritis
- IIF:
-
indirect immunofluorescence
- HLE:
-
human leukocyte elastase
- GBM:
-
glomerular basement membrane
- IgAN:
-
IgA nephropathy
- HSP:
-
Henoch-Schönlein purpura
References
Bories D, Raynal MC, Solomon DH et al. (1989) Downregulation of a serine protease, myelobastin, causes growth arrest and differentiation of promyelocytic leukemia cells. Cell 59:959–968
Brown WJ, Shannon WA Jr, Snell WJ (1983) Specific and azurophilic granules from rabbit polymorphonuclear leukocytes. III: Cell surface localization of granule membrane and content proteins before and after degranulation. J Cell Biol 96:1040–1046
Cohen Tervaert JW, Van der Woude FJ, Fauci AS, Ambrus JL, Velosa J, Keane WF, Meijer S, Van der Giessen M, The TH, Van der Hem GK, Kallenberg CGM (1989) Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med 149:2461–2465
Cohen Tervaert JW, Goldschmeding R, Elema JD, Limburg PC, Van der Giessen M, Huitema MG, Koolen MI, Hené RJ, The TH, Van der Hem GK, Von dem Borne AEGK, Kallenberg CGM (1990 a) Association of autoantibodies to myeloperoxidase with different forms of vasculitis. Arthritis Rheum 33:1264–1272
Cohen Tervaert JW, Goldschmeding R, Elema JD et al. (1990b) Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int 37:799–806
Davies DJ, Moran JE, Nial JF, Ryan GB (1982) Segmental necrotizing glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology. BMJ 265:606
Dolman KM, Goldschmeding R, Sonnenberg A et al. (1990) ANCA related antigens. Acta Pathol Microbiol Immunol Scand 98:28
Falk RJ, Jennette JC (1988) Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 318:1651–1657
Fauci AS, Haynes BF, Katz P (1978) The spectrum of vasculitis: clinical, pathological, immunologic, and therapeutic considerations. Ann Intern Med 89:660–676
Flesch BK, Lampe M, Rautman A et al. (1991) Anti-elastase cathepsin G and lactoferrin antibodies in sera with c-ANCA or with atypical fluorescence staining pattern. In: The Third International Workshop on ANCA. Washington, November 1990 (abstr). Am J Kidney Dis (in press)
Goldschmeding R, van der Schoot CE, ten Bokkel Huinink D et al. (1989) Wegener's granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysomes of normal human neutrophils. J Clin Invest 84:1577–1587
Gupta SK, Niles JL, McCluskey RT et al. (1990) Identity of Wegener's autoantigen (p29) with proteinase 3 and myeloblastin. Blood 76:2162
Hall JB, Wadham BMCN, Wood JC et al. (1984) Vasculitis and glomerulonephritis: a subgroup with an antineutrophil cytoplasmic antibody. Aust NZ J Med 14:277–278
Jenne DE, Tschopp J, Lüdemann J et al. (1990) Wegener's autoantigen decoded. Nature 346:520
Jennette JC, Hoidal JH, Falk RJ (1990) Specificity of antineutrophil cytoplasmic autoantibodies for proteinase 3. Blood 75:2263–2264
Johnson RJ, Couser WG, Chi EY et al. (1987) New mechanisms for glomerular injury. Myeloperoxidase-hydrogen peroxide —halide system. J Clin Invest 79:1379–1387
Johnson RJ, Couser WG, Alpers CE et al. (1988a) The human neutrophil serine proteinases, elastase and cathepsin G, can mediate glomerular injury in vivo. J Exp Med 168:1169–1174
Johnson RJ, Guggenheim SJ, Klebanoff SJ et al. (1988) Morphologic correlates of glomerular oxidant injury induced by the myeloperoxidase —hydrogen peroxide -halide system of the neutrophil. Lab Invest 58:294–301
Kao RC, Wehner NG, Skubitz KM et al. (1988) Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamster. J Clin Invest 82:1963–1973
Lie JT (1987) The classification and diagnosis of vasculitis in large and medium-sized blood vessels. Pathol Ann 22:125–162
Lüdemann J, Utecht B, Gross WL (1990) Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J Exp Med 171:357–362
Nässberger L, Jonsson H, Sjöholm AG et al. (1989) Circulating anti-elastase in systemic lupus erythematosus. Lancet I (letter):509
Niles JL, McCluskey RT, Ahmad MF et al. (1989) Wegener's granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood 74:1888–1893
Nölle B, Specks V, Lüdermann J, Rohrbach MS, De Remee RA, Gross WL (1989) Anticytoplasmic autoantibodies: their immunodiagnostic value in Wegener's granulomatosis. Ann Intern Med 111:28–40
O'Donoghue D, Nusbaum P, Halbwachs-Mecarelli L, Lesavre P (1991a) Antimyeloperoxidase antibodies of IgG isotype in IgA nephropathy (abstract). Am J Kidney Dis (in press)
O'Donoghue P, Vanhille P, Lesavre P, Noël LH (1991 b) Antimyeloperoxidase antibodies in antiglomerular basement membrane disease (abstract). Am J Kidney Dis (in press)
Pereira A, Spitznagel J, Winton EF et al. (1990) The ontogeny of a 57-kD cationic antimicrobind protein of human polymorphonuclear leukocytes: localization to a novel granule population. Blood 76:825–834
Pozzi C, Radice A, Rota S et al. (1991) Clinical significance of anti-lactoferrin antibodies in renal disease. In: The Third International Workshop on ANCA. Washington, November 1990 (abstract). Am J Kidney Dis (in press)
Savage COS, Winearls CG, Evans DJ, Rees AJ, Lockwood CM (1985) Microscopic polyarteritis: presentation, pathology and prognosis. Q J Med 56:467–483
Shah SV, Baricos WH, Basci A (1987) Degradation of human glomerular basement membrane by stimulated neutrophils. Activation of a metallo-proteinase(s) by reactive oxygen metabolites. J Clin Invest 79:25–31
Thomson RA, Lee SS (1989) Antineutrophil cytoplasmic antibodies. Lancet I (letter):670–671
Van der Woude FJ, Daha MR, van Es LA (1989) The current status of neutrophil cytoplasmic antibodies. Clin Exp Immunol 78:143–148
Wiik A (1989) Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. APMIS 97 (Supp l6):12
Wiik A, Van der Woude FJ (1990) The new ACPA/ANCA nomenclature. Neth J Med 36:107–108
Wilde CG, Snable JL, Griffith JE et al. (1990) Characterization of two azurophil granule proteases with active site homology to neutrophil elastase. J Biol Chem 265:2038–2041
Author information
Authors and Affiliations
Additional information
Preprint of a lecture to be read at the 22nd Congress of the “Gesellschaft für Nephrologie”, Heidelberg, September 15–18, 1991 (Editor: Prof. Dr. E. Ritz, Heidelberg)
Rights and permissions
About this article
Cite this article
Lesavre, P., Noël, L.H., Chauveau, D. et al. Antigen specificities and clinical distribution of ANCA in kidney diseases. Klin Wochenschr 69, 552–557 (1991). https://doi.org/10.1007/BF01649317
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01649317