Summary
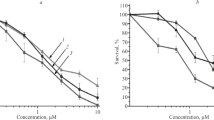
The colony formation in agar of human tumor xenografts, of murine tumors and of human bone marrow was used as a test system to determine the in vitro activity of the two novel cytostatic agents, mitozolomide and sparsomycin. Mitozolomide was additionally studied in vivo in nine human tumor xenografts. The comparison of in vitro/in vivo activity allows an assessment of the relevant in vitro dose based on in vivo pharmacological behavior of a compound. Both compounds showed clear dose/response effects in vitro. A dose of 3 μg/ml mitozolomide, given by continuous exposure, was active (colony number of test <30% of the control group) in 12/42 (29%) human tumor xenografts as well as in the four murine tumors, P388, L1210, B16 melanoma and colon carcinoma 38, whereas the two human bone marrows showed no significant suppression of the ability to form colonies in culture. The comparison of in vitro with in vivo activity suggests that the in vitro dose of 3 μg/ml corresponds best to the activity observed in animal experiments. The highest activity was observed in small-cell cancer of the lung (4/5), followed by melanomas (2/7) and non-small-cell cancer of the lung (2/9). Furthermore, activity was found in a cancer of the large bowel, stomach, breast and in one sarcoma. In the treatment of nine human tumor xenografts growing subcutaneously in nude mice, mitozolomide effected a complete or partial remission in 6 out of 9 tumors. In comparison to standard drugs mitozolomide is one of the most effective compounds in these tumors. These data indicate that mitozolomide possesses potent broad-spectrum activity in human tumor xenografts. Sparsomycin (0.1 μg/ml, continuous exposure) was active in 11/46 (24%) human tumor xenografts and in 4/5 of the murine tumors, whereas the colony-forming capacity of four human bone-marrows showed no inhibition, suggesting that this dose level may be the relevant in vitro dose. However, the high in vitro activity in murine tumors is incompatible with the in vivo activity. In mice the only responsive tumor was leukemia P388, whereas the L1210, B16 melanoma and colon carcinoma 38 were resistant. At the dose level of 0.03 μg/ml only 3/30 (10%) of the human tumor xenografts were sensitive. In an earlier clinical phase I study the dose-limiting adverse effect was eye toxicity and not bone-marrow suppression. This example illustrates that comparing in vitro with in vivo activity in the same tumor results in a more reliable estimation of the relevant in vitro dose than does comparing in vitro activity with in vitro effects on human bone marrow.
Similar content being viewed by others
Abbreviations
- Mitozolomide:
-
8-carbamoyl-3-(2-chloroethyl)imidazo(5,1-d)-1,2,3,5-tetrazin-4(3H)-one
- NSC 353451:
-
formerly known as azolastone
- sparsomycin:
-
NSC 59 729
- DTIC:
-
5-(3,3-dimethyltriazen-1-yl)-imidazole-4-carboxamide
- MTIC:
-
5-(3-methyltriazen-1-yl)imidazole-4-carboxamide
- DMSO:
-
dimethylsulfoxide
References
Alley MC, Uhl CB, Lieber MM (1982) Improved detection of drug cytotoxicity in the soft agar colony formation assay through use of a metabolizable tetrazolium salt. Life Sci 31:3071–3078
Carney DN, Winkler CF (1985) In vitro assays of chemotherapeutic sensitivity. In: VT DeVita, S Hellman, and SA Rosenberg (eds) Important advances in oncology Lippincott, Philadelphia pp 78–103
Close HP, McFarlane JR (1964) Ocular toxicity with sparsomycin (NSC 59729) in a phase I study: a preliminary report. Cancer Chemother Rep 43:29–31
Fiebig HH (1988) Comparison of tumor response in nude mice and in the patients. In: Winograd B, Peckham MJ, Pinedo HM (eds) Human tumor xenografts in anticancer drug development. Springer, Berlin Heidelberg New York, pp 25–30
Fiebig HH, Widmer KH, Fiedler L, Wittekind C, Löhr GW (1984) Development and characterization of 51 human tumor models for large bowel, stomach and esophageal cancers. Prog Rep Dig Surg 1:225–235
Fiebig HH, Neumann H, Henß H, Koch H, Kaiser D, Arnold H (1985a) Development of 3 human small cell lung cancer models in nude mice. Rec Results Cancer Res 97:77–86
Fiebig HH, Schmid JR, Henß H, Dentier U, Schildge J, Löhr GW (1985b) Bedeutung des Kolonie-Assays als in-vitro Verfahren zur Tumorsensibilitätstestung und für die Zytostatikaentwicklung. In: P Drings et al (eds) Bronchialkarzinom. Bandreihe Aktuelle Onkologie. W Zuckschwerdt, München
Fiebig HH, Schmid JR, Bieser W, Henss H, Löhr GW (1987) Colony assay with human tumor xenografts, murine tumors and human bone marrow. Potential for anticancer drug development. Eur J Cancer Clin Oncol 23:937–948
Fiebig HH, Winterhalter BR, Berger DP, Löhr GW (1989a) Combined in vitro/in vivo test procedure with human tumor xenografts for anticancer drug development. Strahlenther Onkol 165:522–524
Fiebig HH, Widmer KH, Winterhalter BR, Löhr GW (1989b) CGP 6809 — a new nitrosoureido-sugar derivative with activity in human tumor xenografts. Cancer Chemother Pharmacol 23:337–340
Fodstad O, Aamdal S, Pihl A, Boyd MR (1985) Acitivity of Mitozolomide (NSC 353451), a new imidazotetrazine, against xenografts from human melanomas, sarcomas, and lung and colon carcinomas. Cancer Res 45:1778–1786
Fortmeyer HP (1981) Thymusaplastische Maus (nu/nu) — thymusaplastische Ratte (rnu/rnu) — Haltung, Zucht, Versuchsmodelle. Schriftenreihe Versuchstierkunde 8. P Parey, Berlin
Gibson NW, Erickson LC, Hickman JA (1984) Effects of the antitumor agent 8-carbamoyl-3-(2-chloroethyl)imidazo(5,1-d)-1,2,3,5-tetrazin-4(3H)-one on the DNA of mouse L1210 cells. Cancer Res 44:1767–1771
Hamburger AW, Salmon SE (1977) Primary bioassay of human tumor stem cells. Science 197:461–463
Hickman JA, Stevens MFG, Gibson NW, Langdon SP, Fizames C, Lavelle F, Atassi G, Lunt E, Tilson RM (1985) Experimental antitumor acitivity against murine tumor model systems of 8-carbamoyl-3-(2-chloroethyl)imidazo(5,1-d)-1,2,3,5-tetrazin-4(3H)-one (mitozolomide), a novel broad-spectrum agent. Cancer Res 45:3008–3013
Horgan CMT, Tisdale MJ (1984) Antitumor imidazotetrazines. An investigation into the mechanism of antitumor activity of a novel and potent antitumor agent CCRG 81010 (M&B 39565; NSC 354451). Biochem Pharmacol 33:2185–2192
National Cancer Institute data base, Washington
Neumann HA, Fiebig HH, Engelhardt R, Löhr GW (1985) Cytostatic drug effects on human clonogenic tumor cells and human bone marrow progenitor cells (CFU-C) in vitro. Res Exp Med 185:51–56
Owen SP, Dietz A, Camiener GW (1962) Sparsomycin, a new antitumor antibiotic. I. Discovery and biological properties. Antimicrob Agents 772–779
Ottenheijm HCJ, Liskamp RMJ, van Nispen SPJM, Boots HA, Tyhuis MW (1981) The total synthesis of the antibiotic Sparsomycin, a modified uracil amido acid monoxodithioacetal. J Org Chem 46:3273–3283
Salmon SE (1984) Human tumor colony assay and chemosensitivity testing. Cancer Treat Rep 68:117–125
Schlunk T, Schleyer M (1980) The influence of culture conditions on the production of colony-stimulating activity by human placenta. Exp Hematatol 8:179–184
Shoemaker RH, Wolpert-DeFilippes MK, Venditti JM (1984) Potentials and drawbacks of the human tumor stem cell assay. Behring Inst Mitt 74:262–272
Shoemaker RH, Wolpert-DeFilippes MK, Kern DH, Lieber MM, Makuch RW, Melnick NR, Miller WT, Salmon SE, Simon RM, Venditti JM, Von Hoff DD (1985) Application of a human tumor colony-forming assay to new drug screening. Cancer Res 45:2145–2153
Stevens MFG, Hickman JA, Stone R, Gibson NW, Lunt E, Newton CG, Baig GU (1984) Antitumor imidazotetrazines. 1. Synthesis and chemistry of 8-carbamoyl-3-(2-chloroethyl)imidazo(5,1-d)-1,2,3,5-tetrazin-4 (3H)-one, a novel broad-spectrum antitumor agent. J Med Chem 27:196–201
Venditti JM (1981) Preclinical drug development: rational and methods. Semin Oncol 8:349–361
Author information
Authors and Affiliations
Additional information
Dedicated to Professor Dr. D. Schmähl, Heidelberg, on the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Fiebig, H.H., Berger, D.P., Köpping, K. et al. In vitro and in vivo anticancer activity of mitozolomide and sparsomycin in human tumor xenografts, murine tumors and human bone marrow. J Cancer Res Clin Oncol 116, 550–556 (1990). https://doi.org/10.1007/BF01637073
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01637073