Summary
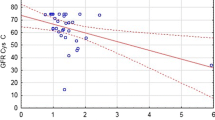
In order to detect even subclinical hints of nephrotoxicity after application of carboplatin, sensitive non-invasive methods, e.g. determination of urinary enzyme (lactate dehydrogenase, leucine aminopeptidase,γ-glutamyltransferase,N-acetyl-β-glucosaminidase), glomerular and tubular protein excretion (albumin,α-1-microglobulin) and determination of the creatinine clearance, were used. Eighteen patients with small-cell lung cancer entered the study. All patients were treated with the three-drug combination chemotherapy: vincristine (1.5 mg i.v. on days 1, 8, 15, 22), etopside (escalating doses: 100–160 mg/m2 on days 1–3) and carboplatin (300 mg/m2 day 1). Investigations were made during the first, third and fifth treatment cycles. Deterioration of renal function occurred in 4 out of 18 patients in all three observed treatment courses. Abnormal amounts in the excretion of at least one of four urinary enzymes were found in 6 out of 18 patients during the first cycle and in 4 out of 8 patients during the third and fifth cycles. All patients with pathological enzymuria during the first treatment course also developed an increased enzymuria during cycles 3 and 5. Four patients developed pathological proteinuria during the first and 2 patients during the third and fifth cycles. These findings demonstrate that the new platinum analogue, carboplatin, is capable of inducing renal damage. In comparison with cisplatinum, the nephrotoxicity of this new analogue is reduced but not completely eliminated.
Similar content being viewed by others
References
Buamah PK, Howell A, Whitby H, Harpur S, Gescher A (1982) Assessment of renal function during high-dose cis-platinum therapy in patients with ovarian carcinoma. Cancer Chemother Pharmacol 8:281–284
Calvert AH, Harland SJ, Newell DR, Siddik ZH, Jones AC, McElwain TJ, Raju S, Wiltshaw E, Smith IE, Baker JM, Peckham MJ, Harrap KR (1982) Early clinical studies with cis-diammine-1,1-cyclobutane dicarboxylate platinum. II. Cancer Chemother Pharmacol 9:140–147
Calvert AH, Horwich A, Newlands ES, Begent R, Rustin GJS, Kaye SB, Harris AL, Williams CJ, Slevin L (1988) Carboplatin or cisplatin? Lancet 2:577–578
Canetta R, Rozencweig M, Carter SK (1985) Carboplatin: the clinical spectrum to date. Cancer Treat Rev 12:125–136
Cohen AI, Harberg J, Citrin DL (1981) Measurement of urinaryβ2-microglobulin in the detection of cis-platin nephrotoxicity. Cancer Treat Rep 65:1083–1085
Curt AG, Grygiel JJ, Corden BJ, Ozols RF, Weiss RB, Tell DT, Myers CE, Collins JM (1983) A phase I and pharmacokinetic study of diamminecyclobutanedicarboxylatoplatin (NSC 241240). Cancer Res 43:4470–4473
Diener U, Knoll E, Langer B, Rautenstrauch H, Ratge B, Wisser H (1981) Urinary excretion ofN-acetyl-β-glucosaminidase and alanine aminopeptidase in patients receiving amikacin or cis-platinum. Clin Chim Acta 112:149–157
Gore ME, Calvert AH, Smith IE (1987) High-dose carboplatin in the treatment of lung cancer and mesothelioma: a phase I dose escalating study. Eur J Cancer Res Clin Oncol 23:1391–1397
Groth S, Nielsen H, Sorensen B, Christensen B, Pedersen AG, Rorth M (1986) Acute and longterm nephrotoxicity ofcis-platinum in man. Cancer Chemother Pharmacol 17:191–196
Harland SJ, Newell DR, Siddik ZH, Chadwick R, Calvert AH, Harrap KR (1984) Pharmacokinetics of cis-diammine-1,1-cyclobutane dicarboxylate platinum (II) in patients with normal and impaired renal function. Cancer Res 44:1693–1697
Jones BR, Bhalla RB, Mladek J, Kalcya RN, Gralla RJ, Alcock NW, Schwartz MK, Young CW, Rcidcnberg NW (1980) Comparison of methods of evaluating nephrotoxicity of cis-platinum. Clin Pharmacol Ther 4:557–562
Maruhn D, Fuchs I, Mues G (1976) Normal limits of urinary excretion of eleven enzymes. Clin Chem 22:1567–1574
Maruhn D, Strozyk K, Gielow L (1977) Diurnal variations of urinary enzyme excretions. Clin Chim Acta 75:427–433
Metz U, Kurschel E, Graben N (1985) Monitoring renal toxicity of cis-platinum by urinary enzymes. Proc EDTA 22:1087–1090
Mitrou PS, Mondorf W (1979) Cis-Platin-Ncphrotoxizität: Effekt auf die proximalen Nierentubuli gemessen an der Ausscheidung der Alaninaminopeptidase(AAP) im Urin. In: Seeber S, Schmidt CG, Nagel G, Achterrath W (Hrsg) Cisplatin derzeitiger Stand und neue Entwicklungen in der Chemotherapie maligner Neoplasien. Karger, Basel München Paris London New York Sidney, pp 34–44
Mulder POM, Sleijfer DT, de Vries EGE, Uges DRA (1988) Renal dysfunction following high-dose carboplatin treatment. Eur J Cancer Res Clin Oncol 114:211–214
Osman N, Copley MP, Litterst CL (1984) Effects of the diuretics mannitol or acetazolamide on the nephrotoxicity and physiological disposition of cisplatin in rats. Cancer Chemother Pharmacol 13:58–62
Ozols RF, Jacob J, Welsley MN, Ostega Y, Young RC (1984) High-dose Cisplatin in hypertonic saline. Ann Int Med 100:19–24
Rose WC, Schurig JE (1985) Preclinical antitumor and toxicological profile of carboplatin. Cancer Treat Rev 12:1–19
Skillen AW, Buamah PK, Cantwell BNJ, Cornell C, Hodson AW, Harris AL (1988) Urinary protein and enzyme excretion in patients receiving chemotherapy with thecis-platinum analogs carboplatin (CBDCA, JM8) and iproplatin (CHIP, JM 9). Cancer Chemother Pharmacol 22:228–234
Van Echo DA, Egorin MJ, Whitacre MY, Olman EA, Aisner J (1984) Phase I clinical and pharmacological trial of carboplatin for 5 days. Cancer Treat Rep 68:1103–1114
Vogl SE, Zaravinos T, Kaplan BH, Wollner D (1981) Safe and effective two-hour regimen of hydration and diuresis for the administration of cis-diamminedichloroplatinum (II). Eur J Cancer 17:345–350
Von Hoff DD (1987) Whither carboplatin? — A replacement for an alternative to cis-platin? J Clin Oncol 2:169–171
Werner M, Maruhn D, Atoba M (1969) Use of gel filtration in the assay of urinary enzymes. J Chromatogr 40:254–257
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Metz-Kurschel, U., Kurschel, E., Niederle, N. et al. Investigations on the acute and chronic nephrotoxicity of the new platinum analogue carboplatin. J Cancer Res Clin Oncol 116, 203–206 (1990). https://doi.org/10.1007/BF01612678
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01612678