Summary
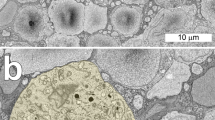
With indirect immunofluorescence, glutamate decarboxylase (GAD), the GABA synthesizing enzyme, was localized to cell bodies in the inner half of the inner nuclear layer and a few in the outer tier of the ganglion cell layer in the rhesus monkey retina. In the inner plexiform layer there were three strongly GAD-immunoreactive laminae separated by two less immunoreactive laminae. Electron microscopy demonstrated that the GAD was contained in amacrine cells and these GAD-immunoreactive amacrines were primarily pre- and postsynaptic to biopolar cell axon terminals. The GAD-containing processes possessed small synaptic vesicles and formed synapses that could be characterized as symmetrical. Large, dense-cored vesicles were often found in the cell bodies and synaptic processes of the GAD-immunoreactive amacrine cells. As the vast majority of the synaptic input and output of the GAD-containing amacrine cells was to and from bipolar cells and the strongest GAD-immunoreactivity correlated with the endings of bipolar cells that connect with a single cone, the functional effects of GABA in the primate retina are likely to be found in the responses of single cone pathways in the inner plexiform layer.
Similar content being viewed by others
References
Allen, R. A. (1969) The retinal bipolar cells and their synapses in the inner plexiform layer. InThe Retina: Morphology, Function and Clinical Characteristics (edited byStraatsma, B. R., Hall, M. O., Allen, R. A. &Crescitelli, F.), pp. 101–43. Los Angeles: University of California Press.
Boycott, B. B. &Dowling, J. E. (1969) Organization of the primate retina: light microscopy.Philosophical Transactions of the Royal Society of London, Series B255, 109–76.
Brandon, C. (1985) Retinal GABA neurons: localization in vertebrate species using an antiserum to rabbit brain glutamate decarboxylase.Brain Research 344, 286–95.
Brandon, C., Lam, D. M. K. &Wu, J. Y. (1979) The gamma-aminobutyric acid system in rabbit retina: localization by immunocytochemistry and autoradiography.Proceedings of the National Academy of Sciences USA 76, 3557–61.
Cajal, S. R. y (1933) La retine des vertebres. InThe Structure of the Retina (translated byThorpe, S. A., &Glickstein, M., 1972). Springfield, Illinois: Charles C. Thomas.
Chan-Palay, V., Nilaver, G., Palay, S. L., Beinfeld, M. C., Zimmerman, E. A., Wu, J. Y. &O'Donohue, T. L. (1981) Chemical heterogeneity in cerebellar Purkinje cells: existence and coexistence of glutamic acid decarboxylase-like and motolin-like immunoreactivities.Proceedings of the National Academy of Sciences USA 78, 7787–91.
Coons, A. H. (1958) Fluorescent antibody methods. InGeneral Cytochemical Methods (edited byDanielli, J. F.), pp. 399–422. New York: Academic Press.
Colonnier, M. (1968) Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study.Brain Research 9, 268–87.
Dowling, J. E. &Boycott, B. B. (1966) Organization of the primate retina: electron microscopy.Proceedings of the Royal Society of London, Series B 166, 80–111.
Ehinger, B. (1970) Autoradiographic identification of rabbit retinal neurons that take up GABA.Experientia 26, 1063–4.
Famiglietti, E. V. Jr &Kolb, H. (1975) A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina.Brain Research 84, 293–300.
Gilok, H. &Sedat, J. W. (1982) Fluorescence microscopy: reduced photobleaching of rhodamine and fluorecein in protein conjugates by n-propyl gallate.Science 217, 1252–5.
Gray, E. G. (1959) Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study.Journal of Anatomy 93, 420–33.
Hendrickson, A., Ryan, M., Noble, B. &Wu, J. Y. (1985) Colocalization of3(H)muscimol and antisera to GABA and glutamic acid decarboxylase within the same neurons in monkey retina.Brain Research 348, 391–6.
Kosaka, T., Hataguchi, Y., Hama, K., Nagatsu, I. &Wu, J. Y. (1985) Coexistence of immunoreactivities for glutamate decarboxylase and tyrosine hydroxylase in some neurons in the periglomerular region of the rat main olfactory bulb: possible coexistence of gammaaminobutyric acid (GABA) and dopamine.Brain Research 343, 166–71.
Mariani, A. P. (1981) A diffuse invaginating cone bipolar cell in primate retina.Journal of Comparative Neurology 197, 661–71.
Mariani, A. P. (1982a) ‘Association’ amacrine cells could mediate directional selectivity in pigeon retina.Nature 298, 654–5.
Mariani, A. P. (1982b) Biplexiform cells: ganglion cells of the primate retina that contact photoreceptors.Science 216, 1134–6.
Mariani, A. P. (1983a) A morphological basis for verticality detectors in the pigeon retina: asymmetric amacrine cells.Naturwissenschaften 70, 368–9.
Mariani, A. P. (1983b) Giant bistratified bipolar cells in monkey retina.Anatomical Record 206, 215–20.
Mariani, A. P. (1984a) Bipolar cells in monkey retina selective for the cones likely to be blue sensitive.Nature 308, 185–6.
Mariani, A. P. (1984b) The neuronal organization of the outer plexiform layer of the primate retina.International Review of Cytology 86, 285–320.
Mariani, A. P., Kolb, H. &Nelson, R. (1984) Dopamine-containing amacrine cells of rhesus monkey retina parallel rods in spatial distribution.Brain Research 322, 1–7.
Mosinger, J. L. &Yazulla, S. (1985) Colocalization of GAD-like immunoreactivity and3H-GABA uptake in amacrine cells of rabbit retina.Journal of Comparative Neurology 240, 396–406.
Nelson, R. (1982) AII amacrine cells quicken time course of rod signals in the cat retina.Journal of Neurophysiology 47, 928–47.
Nishimura, Y., Schwartz, M. L. &Rakic, P. (1985) Localization of γ-aminobutyric acid and glutamic acid decarboxylase in rhesus monkey retina.Brain Research 359, 351–5.
Ohara, P. T., Lieberman, A. R., Hunt, S. P. &Wu, J.Y. (1983) Neural elements containing glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the rat.Neuroscience 8, 189–211.
Oertel, W. H., Graybiel, A. M., Mugnaini, E., Elde, R. P., Schmechel, D. E. &Kopin, I. J. (1983) Coexistence of glutamic acid decarboxylase- and somatostatin-like immunoreactivity in neurons of the feline nucleus reticularis thalami.Journal of Neuroscience 3, 1322–32.
Oertel, W. H., Schmechel, D. E., Mugnaini, E., Tappaz, M. L. &Kopin, I. J. (1981) Immunocytochemical localization of glutamate decarboxylase in rat cerebellum with a new antiserum.Neuroscience 6, 2715–35.
Polyak, S. L. (1957) Structure of the retina. InThe Vertebrate Visual System, pp. 207–87. Chicago: University of Chicago Press.
Pourcho, R. G. (1980) Uptake of (2H)glycine and (3H)GABA by amacrine cells in the cat retina.Brain Research 198, 333–46.
Sternberger, L. (1979)Immunocytochemistry. New York: Wiley.
Vallerga, S. &Deplano, S. (1984) Differentiation, extent and layering of amacrine cell dendrites in the retina of a sparid fish.Proceedings of the Royal Society of London, Series B221, 465–77.
Vaney, D. I. (1985) The morphology and topographic distribution of All amacrine cells in the cat retina.Proceedings of the Royal Society of London, Series B224, 475–88.
Vaughn, J. E., Famiglietti, E. V. Jr, Barber, R. P., Saito, K., Roberts, E. &Ribak, C. E. (1981) GABAergic amacrine cells in rat retina: immunocytochemical identification and synaptic connectivity.Journal of Comparative Neurology 197, 113–27.
Venable, J. H. &Coggeshall, R. (1965) A simplified lead citrate stain for use in electron microscopy.Journal of Cell Biology 25, 407–8.
Wong-Riley, M. T. T. (1974) Synaptic organization of the inner plexiform layer in the retina of the tiger salamander.Journal of Neurocytology 3, 1–33.
Wood, J. G., McLaughlin, B. J. &Vaughn, J. E. (1976) Immunocytochemical localization of GAD in electron microscopic preparations of rodent CNS. InGABA in Nervous System Function (edited byRoberts, E., Chase, T. N. &Tower, D. B.), pp. 133–48. New York: Raven Press.
Zucker, C., Yazulla, S. &Wu, J. Y. (1984) Noncorrespondence of (3H)GABA uptake and GAD localization in goldfish amacrine cells.Brain Research 298, 154–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mariani, A.P., Caserta, M.T. Electron microscopy of glutamate decarboxylase (GAD) immunoreactivity in the inner plexiform layer of the rhesus monkey retina. J Neurocytol 15, 645–655 (1986). https://doi.org/10.1007/BF01611863
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01611863