Abstract
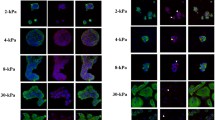
Stromal-epithelial interactions as expressed by alpha-smooth muscle actin (alpha-SM actin) collagen type IV, fibronectin, laminin and tenascin were studied in normal and pathological endometrial mucosa. There was complete or incomplete cuffing of scattered endometrial glands by alpha-SM actin mostly in the basal layer of endometrial mucosa and in dilated or cystic glands. Individual adenocarcinomatous glands were not encircled by alpha-SM actin-positive cells. Collagen type IV and laminin were found surrounding all glands irrespective of the presence of periglandular alpha-SM actin. Fibronectin was diffusely present in the stroma. Tenascin was identified, albeit not exclusively, in a periglandular location similar to that of alpha-SM actin. We conclude that the periglandular cells staining for alpha-SM actin, which were negative for cytokeratins, are probably myofibroblasts (MFs). Since this phenomenon was most commonly observed in dilated and cystic glands, we suggest that stromal cells immediately surrounding these glands may be subjected to mechanical or other stress resulting, as in other situations of tissue remodelling, in the development of MFs. This may also explain the appearance of tenascin in the same location. Thus, our finding may represent a further example of the local modulation of stromal cell phenotype, possibly under the action of micro-environmental factors.
Similar content being viewed by others
References
Aggarwal BB, Pocsik E (1992) Cytokines: from clone to clinic. Arch Biochem 292:335–359
Barcellos-Hoff MH, Aggeler J, Ram TG, Bissei MJ (1989) Functional differentiation and alveolar morphogenesis of primary mammary basement membrane. Development 105:223–235
Bo WJ, Odor DL, Rockvock MF (1968) Ultrastructure of uterine smooth muscle following progesterone or progesterone-estrogen treatment. Anat Rec 163:121–132
Bussolati GC (1980) Actin-rich (myoepithelial) cells in lobular carcinoma in situ of the breast. Virchows Arch [B] 32:165–176
Cintorino M, Bellizzi de Marco E, Leoncini P, Tripodi SA, Ramaekers F, Sappino AP, Schmitt-Gräff A, Gabbiani G (1991) Expression of alpha-smooth muscle actin in stromal cells of the uterine cervix during epithelial neoplastic changes. Int J Cancer 47:843–846
Czernobilsky B, Shezen E, Lifschitz-Mercer B, Fogel M, Luzon A, Jacob N, Skalli O, Gabbiani G (1989) Alpha-smooth muscle actin (alpha-SM actin) in normal human ovaries, in ovarian stromal hyperplasia and in ovarian neoplasms. Virchows Arch [B] 57:55–61
Desmoulière A, Rubbia-Brandt L, Abdiu A, Walz T, Macieira-Coelho A, Gabbiani G (1992)α-smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated byγ-interferon. Exp Cell Res 201:64–73
Desmoulière A, Rubbia-Brandt L, Grau G, Gabbiani G (1992) Heparin inducedα-smooth muscle actin expression in cultured fibroblasts and in granulation tissue myofibroblasts. Lab Invest 67:716–726
Donjacour AD, Cunha GR (1991) Stromal regulation of epithelial function. In: Lipmann M, Dickson R (eds) Regulatory mechanisms in breast cancer. Kluwer Academic, Boston, pp 335–364
Franke WW, Appelhans B, Schmid E, Freudenstein C, Osborn M, Weber K (1979) Identification and characterization of epithelial cells in mammalian tissues by immunofluorescence microscopy using antibodies to prekeratin. Differentiation 15:7–25
Gabbiani G, Ryan GB, Majno G (1971) Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27:549–550
Haffen K, Kedinger M, Simon-Assmann P (1987) Mesenchyme-dependent differentiation of epithelial progenitor cells in the gut. J Pediatr Gastroenterol Nutr 6:14–23
Hodges GM (1982) Tumor formation: the concept of tissue (stromal-epithelium) regulatory dysfunction. In: Pitts JD, Finbow ME (eds) The functional integration of cells in animal tissues. Cambridge University Press, Cambridge, pp 333–356
Howeedy AA, Virtanen I, Laitinen L, Gould NS, Koukoulis GK, Gould VE (1990) Differential distribution of tenascin in the normal, hyperplastic, and neoplastic breast. Lab Invest 63:798–806
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedure. J Histochem Cytochem 29:577–580
Kawaguchi K, Fujii S, Konishi I, Okamura H, Mori T (1985) Ultrastructural study of cultured smooth muscle cells from uterine leiomyoma and myometrium under the influence of sex steroids. Gynecol Oncol 21:32–41
Kocher O, Skalli O, Bloom WS, Gabbiani G (1984) Cytoskeleton of rat aortic smooth muscle cells: Normal conditions and experimental intimai thickening. Lab Invest 50:645–652
Kratochwil K (1986) The stroma and the control of cell growth. J Pathol 149:23–24
Liotta LA, Roa CN, Werwer UM (1986) Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem 55:1037–1057
Liotta LA, Steeg PS, Stettier-Stevenson G (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64:327–336
Mackie EJ, Halfter W, Liverani D (1988) Induction of tenascin in healing wounds. J Cell Biol 107:2257–2767
Montesano R, Schaller G, Orci L (1991) Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell 66:697–711
Nagle RB, Böcker W, Davin JR, Heid HW, Kaufmann U, Lucas DO, Jarasch ED (1986) Characterization of breast carcinomas by two nomoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem 34:869–881
Nakanishi Y, Ishii T (1989) Epithelial shape change in mouse embryonic submandibular gland: modulation by extracellular matrix components. Bioessays 11:163–167
Nathrath WBJ, Wilson PD, Trejdosiewicz LK (1982) Immunohistochemical localization of keratin and luminal epithelial antigen in myoepithelial and luminal epithelial cells of human mammary and salivary gland tumors. Pathol Res Pract 175:279–288
Ross R, Klebanoff SJ (1967) Fine structural changes in uterine smooth muscle and fibroblasts in response to estrogen. J Cell Biol 32:155–169
Ruoslahti E (1989) Proteoglycans in cell regulation. J Biol Chem 264:13369–13372
Sappino AP, Skalli O, Jackson B, Schürch W, Gabbiani G (1988) Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer 41:707–712
Sappino AP, Dietrich PY, Skalli O, Widgren S, Gabbiani GH (1989) Colonic pericryptal fibroblasts. Differentiation pattern in embryogenesis and phenotypic modulation in epithelial proliferative lesions. Virchows Arch [A] 415:551–557
Sappino AP, Schürch W, Gabbiani G (1990) Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 63:144–161
Schmitt-Gräff A, Gabbiani G (1992) Phenotypic features of stromal cells in normal, premalignant and malignant conditions. Eur J Cancer 28A: 1916–1920
Schürch W, Seemayer TA, Gabbiani G (1992) Myofibroblasts. In: Sternberg SS (ed): Histology for pathologists. Raven Press, New York, pp 109–144
Schalkwijk J, Skejlen PM, Vlimmen-Willems IMJJ van, Oosterling B, Mackie EJ, Verstraeten AA (1991) Tenascin expression in human dermis related to epidermal proliferation. Am J Pathol 139:1143–1150
Seemayer TA, Lagacé R, Schürch W, Tremblay G (1979) Myofibroblasts in the stroma of invasive and metastatic carcinoma: a possible host response to neoplasia. Am J Surg Pathol 3:525–533
Skalli O, Gabbiani G (1988) The biology of the myofibroblast. Relationship to wound contraction and fibrocontractive disease. In: Clark RAF, Henson PM (eds) The molecular and cellular biology of wound repair. Plenum Press, New York, pp 471–496
Van den Hoff A (1988) Stromal involvement in malignant growth. Adv Cancer Res 50:159–196
Vollmer G, Siegal GP, Chiquet-Ehrismann R, Lightner VA, Arnholdt H, Knuppen R (1990) Tenascin expression in the human endometrium and in endometrial adenocarcinomas. Lab Invest 62:725–730
Author information
Authors and Affiliations
Additional information
B. Czernobilsky was on sabbatical leave from the Department of Pathology, Kaplan Hospital, Rehovot, Israel
Rights and permissions
About this article
Cite this article
Czernobilsky, B., Remadi, S. & Gabbiani, G. Alpha-smooth muscle actin and other stromal markers in endometrial mucosa. Vichows Archiv A Pathol Anat 422, 313–317 (1993). https://doi.org/10.1007/BF01608341
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01608341