Abstract
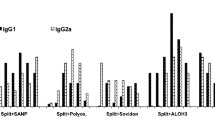
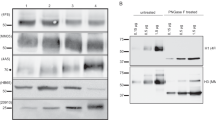
Three split-virion vaccines (Vaxigrip, Begrivac, and Influsplit/Fluarix) and three subunit vaccines containing only viral surface glycoproteins (Influvac, Agrippai, and Fluvirin) available for the 1994–95 season were analysed by biological, molecular, and biochemical methods. Although all vaccines are required by health authorities to contain 15 μg haemagglutinin per dose of each virus strain, there were significant differences in haemagglutination titres among the examined vaccines of both types. The enzymatic activity of neuraminidase was present in all vaccines except Fluvirin. Total protein content was lower for subunit vaccines. Viral nucleoprotein was detected in all split vaccines but to varying levels according to SDS-PAGE and Western blot analyses. The ovalbumin content was low in general but was about tenfold higher for Influvac than for the other vaccines analysed. This protein may induce hypersensitive reactions among persons with severe egg allergy. All three split-virion vaccines were found to contain the matrix protein; however, it was not detected in the subunit vaccines. Differences in influenza antigen variety in currently available vaccines may affect efficacy, whereas differences in concentrations of nonviral compounds such as ovalbumin and endotoxin may lead to different postvaccination reactogenicity profiles.
Similar content being viewed by others
References
Aymard M, Cox NJ, Dubois G, Ghendon Y, Hannoun C, Hampson A, Haaheim LR, Morgan-Capner P, Saliou P, Tamblyn S: Recommendations of the 7th European Meeting of Influenza and Its Prevention. European Journal of Epidemiology 1994, 10: 525–526.
Wiselka M: Influenza: diagnosis, management and prophylaxis. British Medical Journal 1994, 308:1341–1345.
Weeke-Lüttmann M: Licensing and control authority batch release of influenza vaccine in Germany in accordance with EEC regulations. European Journal of Epidemiology 1994, 10: 513–515.
Recommendations of the composition of influenza virus vaccines for the 1994–95 season. World Health Organization Bulletin 1994, 72: 516.
Bradford M: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 1976, 72:248.
Palmer DF, Coleman MT, Dowdle WR, Schild GC: Advanced laboratory techniques for influenza diagnosis. U.S. Department of Health, Education and Welfare. Immunology Series 1975, 6: 34–36.
Lambre CR, Terzedis H, Greffard A, Webster RG: Measurement of anti-influenza neuraminidase antibody using a peroxidase-linked lectin and microtitre plates coated with natural substrates. Journal of Immunological Methods 1990, 135:49–57.
Vaccinum influenzae inactivatum ex virorum fragmentis praeparatum. European Pharmacopoeia Special Print 1994: 1–4.
Vaccinum influenzae inactivatum ex corticis antigeniis praeparatum. European Pharmacopoeia Special Print 1994: 1–3.
Statement on influenza vaccination for the 1994–95 season. Canadian Medical Association Journal 1994, 151:593–597.
Renfrey S, Watts A: Morphological and biochemical characterisation of influenza vaccines commercially available in the United Kingdom. Vaccine 1994, 12: 747–752.
Yankauckas MA, Morrow JE, Parker SE, Abai A, Rhodes GH, Dwarki VJ, Gromkowski SH: Long-term anti-nucleoprotein cellular and humoral immunity is induced by intramuscular injection of plasmid DNA containing NP gene. DNA Cell Biology 1993, 12: 771–776.
Gorse JG, Campbell MJ, Otto EE, Powers DC, Chambers GW, Newman FK: Increased anti-influenza A virus cytotoxic T cell activity following vaccination of the chronically ill elderly with live attenuated or inactivated influenza virus vaccine. Journal of Infectious Diseases 1995, 172:1–10.
Justewicz DM, Doherty PC, Webster RG: The B-cell response in lymphoid tissue of mice immunized with various antigenic forms of the influenza virus hemagglutinin. Journal of Virology 1995, 69: 5414–5421.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chaloupka, I., Schuler, A., Marschall, M. et al. Comparative analysis of six European influenza vaccines. Eur. J. Clin. Microbiol. Infect. Dis. 15, 121–127 (1996). https://doi.org/10.1007/BF01591484
Issue Date:
DOI: https://doi.org/10.1007/BF01591484