Abstract
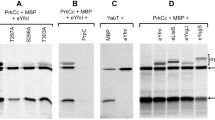
The in vitro phosphorylation of isocitrate lyase was demonstrated in partially purified sonic extracts ofEscherichia coli. Extracts were incubated with [gamma32P]-ATP and subsequently analyzed by two-dimensional polyacrylamide gel electrophoresis. Isocitrate lyase was determined to be phosphorylated by autoradiography and Western blot analyses of the gels. Purified isocitrate lyase comigrates with the phosphorylated form of the enzyme; this suggests that the enzyme may become catalytically active concomitant with phosphorylation.
Similar content being viewed by others
Literature Cited
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Cozzone AJ (1984) Protein phosphorylation in bacteria. Trends Biochem Sci 9:400–403
Garnak M, Reeves HC (1979) Phosphorylation of isocitrate dehydrogenase ofEscherichia coli. Science 203:1111–1112.
Garnak M, Reeves HC (1979) Purification and properties of phosphorylated isocitrate dehydrogenase ofEscherichia coli. J Biol Chem 254:7915–7920
Johnson DA, Gautsch JW, Sportsman JR, Elder JH (1984) Improved technique utilizing nonfat dry milk for analysis of proteins and nucleic acids transferred to nitrocellulose. Gene Anal Tech 1:3–8
Kunce CM, Trelease RN (1986) Heterogeneity of catalase in maturing and germinated cotton seeds. Plant Physiol 81:1134–1139
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 227:680–685
Nimmo HG (1984) Control ofEscherichia coli isocitrate dehydrogenase: an example of protein phosphorylation in a prokaryote. Trends Biochem Sci 9:475–478
O'Farrell PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250:4007–4021
Reeves HC, Malloy PJ (1984) Phosphorylation of isocitrate dehydrogenase inEscherichia coli mutants with a non-functional glyoxylate cycle. FEBS Lett 158:239–242
Robertson EF, Malloy PJ, Ni W, Reeves HC (1986) Isoelectric focusing in vertical polyacrylamide mini-gels. Abstr Annu Meet Am Soc Microbiol 1986:206
Robertson EF, Reeves HC (1986) Purification and characterization of isocitrate lyase fromEscherichia coli. Curr Microbiol 14:347–350
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Walsh K, Koshland DE Jr (1985) Branch point control by the phosphorylation state of isocitrate dehydrogenase. J Biol Chem 260:8430–8437
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robertson, E.F., Hoyt, J.C. & Reeves, H.C. In vitro phosphorylation ofEscherichia coli isocitrate lyase. Current Microbiology 15, 103–105 (1987). https://doi.org/10.1007/BF01589370
Issue Date:
DOI: https://doi.org/10.1007/BF01589370