Summary
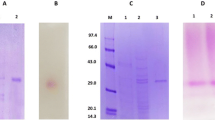
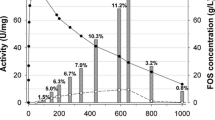
Purification and properties of two β-fructofuranosidases, which produce 1-kestose (1F-β-fructofuranosyl-sucrose) from sucrose, fromAureobasidium sp. ATCC 20524 are reported. The enzymes were purified to homogeneity by fractionations involving ethanol, calcium acetate and ammonium sulfate and DEAE-Cellulofine and Sephadex G-200 chromatography. Molecular weights of the enzymes were estimated to be about 318000 (P-1) and 346000 (P-2) daltons by gel filtration. The enzymes were glycoproteins that contained about 30% (w/v) (P-1) and 53% (w/v) (P-2) carbohydrate. The optimum pH for the enzymatic reactions were 4.5–5.5 (P-1) and 4.5–6 (P-2). The enzymes were stable over a wide pH range (4–9). The optimum reaction temperatures for both enzymes were 50–55°C and they retained more than 94% (P-1) and 98% (P-2) activities at 50°C after 15 min. TheK m values for sucrose were 0.47 M (P-1) and 0.65 M (P-2). The enzymes were inhibited by mercury, copper and lead ions as well asp-chloromercuribenzoate.
Similar content being viewed by others
References
Andrews, P. 1965. The gel-filtration behavior of proteins to their molecular weights over a wide range. Biochem. J. 96: 595–606.
Davis, B.J. 1964. Disk electrophoresis. II. Methods and application to human serum proteins. Ann. N.Y. Acad. Sci. 121: 404–427.
Dubois, M., K.A. Gilles, J.K. Hamilton, P.A. Rebers and F. Smith. 1956. Colorimetric methods for determination of sugar and related substances. Anal. Chem. 28: 350–356.
Fujita, K., K. Hara, H. Hashimoto and S. Kitahata. 1990. Purification and some properties of β-fructofranosidase I fromArthrobacter sp. K-1. Agric. Biol. Chem. 54: 913–919.
Hayashi, S., K. Imada, Y. Kushima and H. Ueno. 1989. Observation of the chemical structure of fructooligosaccharide produced by an enzyme fromAureobasidium sp ATCC 20524. Curr. Microbiol. 19: 175–177.
Hayashi, S., M. Nonokuchi, K. Imada and H. Ueno. 1990. Production of a fructosyl-transferring enzyme byAureobasidium sp. ATCC 20524. J. Ind. Microbiol. 5: 395–400.
Hidaka, H., T. Eida, T. Adachi and Y. Saitoh. 1987. Industrial production of fructooligosaccharides and its application for human and animals. Nippon Nogeikagaku kaishi 61: 915–923.
Hirayama, M., N. Sumi and H. Hidaka. 1989. Purification and properties of a fructooligosaccharide-producing β-fructofuranosidase fromAspergillus niger ATCC 20611. Agric. Biol. Chem. 53: 667–673.
Kitahata, S., S. Suetake and S. Okada. 1986. Purification and transfructosylation reaction of β-fructofuranosidase fromPenicillium sp. K25. Nippon Syokuhin Kogyo Gakkaishi, 33: 826–830.
Lowry, O.H., N.J. Rosebrough, A.L. Farr and R.J. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193: 265–275.
Nishizawa, M., Y. Maruyama and M. Nakamura. 1980. Purification and characterization of invertase isozymes fromFuzarium oxysporum. Agric. Biol. Chem. 44: 489–498.
Yamashita, K., K. Kawai and M. Itakura. 1984. Effects of fructo-oligosaccharides on blood glucose and serum lipids in diabetic subjects. Nutr. Res. 4: 961–966.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hayashi, S., Nonoguchi, M., Takasaki, Y. et al. Purification and properties ofβ-fructofuranosidase fromAureobasidium sp. ATCC 20524. Journal of Industrial Microbiology 7, 251–256 (1991). https://doi.org/10.1007/BF01577652
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01577652