Summary
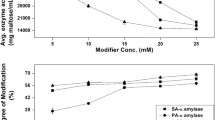
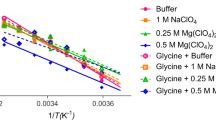
The α-amylase ofBacillus caldovelox is inactivated by diethyl pyrocarbonate at pH 6.6 and 20°C by a monomolecular reaction with a second-order rate constant of 41.7 M−1·min−1. The rate of inactivation increases with decreasing pH, suggesting participation of an amino acid residue with a pK a of 6.6. The increase in absorbance at 240 nm, unchanged absorbance at 280 nm and reactivation in the presence of hydroxylamine suggest the participation of a histidine residue. Statistical analyses of inactivation suggest that only one histidine residue is essential for activity. Substrate afforded complete protection against inactivation, indicating the involvement of the histidine residue at the active site of the enzyme.
Similar content being viewed by others
References
Abdulwajid, A.W. and F.Y.H. Wu. 1986. Chemical modification ofEscherichia coli RNA polymerase by diethyl pyrocarbonate: Evidence of histidine requirement for enzyme activity and intrinsic zinc binding. Biochemistry 25: 8167–8172.
Bealin-Kelly, F., C.T. Kelly, and W.M. Fogarty. 1990. The α-amylase of the caldoactive bacteriumBacillus caldovelox. Biochem. Soc. Trans. 18: 310–311.
Bernfeld, P. 1955. Amylases α-/β-. In: Methods in Enzymology (Colowich, S.P. and N.O. Kaplan, eds.), Vol. 1. pp. 149–158, Academic Press, New York.
Boel, E., L. Brady, A.M. Brzozowski, Z. Derewenda, G.G. Dodson, V.J. Jensen, S.B. Peterson, H. Swift, L. Thim and H.F. Woldlike. 1990. Calcium binding in α-amylases: An X-ray diffraction study at 2.1 Å resolution of two enzymes fromAspergillus. Biochemistry 29: 6244–6249.
Buisson, G., E. Duee, R. Haser and F. Payan. 1987. Three-dimensional structure of porcine pancreatic α-amylase at 2.9 Å resolution. Role of calcium in structure and activity. EMBO J 6: 3909–3916.
Drabikowska, A.K., and G. Wozniak. 1990. Modification of uridine phosphorylase fromEscherichia coli by diethyl pyrocarbonate. Evidence for a histidine residue in the active site of the enzyme. Biochem. J. 270: 319–323.
Fogarty, W.M. 1983. Microbial amylases. In: Microbial enzymes and biotechnology (Fogarty, W.M. ed.), pp. 1–92, Elsevier Applied Science Publishers, Barking, England.
Fogarty, W.M. and C.T. Kelly. 1990. Recent advances in microbial amylases. In: Microbial enzymes and biotechnology, 2nd Edition (Fogarty, W.M., and C.T. Kelly, eds.), pp. 71–132, Elsevier Applied Science Publishers, Barking, England.
Kita, Y., S. Sakaguchi, Y. Nitta and T. Watanabe. 1982. Kinetic study on chemical modification of Taka-amylase A. II. Ethoxycarbonylation of histidine residues. J. Biochem. 92: 1499–1504.
Leatherbarrow, R.J. 1987. Enzfitter, a non-linear regression analysis program for the IBM PC, Elsevier Science Publishers, Amsterdam.
Levy, H.M., H. Leber and E. Ryan. 1963. Inactivation of myosin by 2,4,-dinitrophenol and protection by adenosine triphosphate and other phosphate compounds. J. Biol. Chem. 238: 3654–3659.
Lundblad, R.L. and C.M. Noyes. 1984. Chemical reagents for protein modification. Vols. 1 and 2, CRC Press, Boea Raton, FL, U.S.A.
Matsuura, Y., M. Kusunoki, W. Harada and M. Kakudo. 1984. Structure and possible catalytic residues of Takaamylase A. J. Biochem. 95: 697–702.
Melchoir, W.B. and D. Fahrney. 1970. Ethoxyformylation of proteins. Reaction of ethoxyformic anhydride with α-chymotrypsin, pepsin and pancreatic ribonuclease at pH 4. Biochemistry 9: 251–258.
Miles, E.W. 1977. Modification of hystidyl residues in proteins by diethylpyrocarbonate. In: Methods in Enzymology (Hirs, C.H.W. and S.N. Timasheff, eds.), Vol. 47: 431–453, Academic Press, New York.
Raimbaud, E., A. Buleon, S. Perez and B. Henrissat. 1989. Hydrophobic cluster analysis of the primary sequence of α-amylases. Int. J. Biol. Macromol. 11: 217–225.
Smith, P.K., R.I. Kron, G.T. Hermanson, A.K. Mallia, F.H. Gartner, M.D. Provenzano, E.K. Fujimoto, N.M. Goeke, B.J. Olson and D.C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150: 76–85.
Svensson, B. 1988. Regional distant sequence homology between amylases, α-glucosidases and transglucanosylases. FEBS Lett. 230: 72–76.
Svensson, B., H. Moller and A.J. Clarke. 1988. Chemical modification of carboxyl groups in glucoamylase fromAspergillus niger. Carlsberg Res. Commun. 53: 331–342.
Tsou, C.-L. 1962. Relation between modification of functional groups of proteins and their biological activity. Scientia Sinica, XI: 1535–1558.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bealin-Kelly, F.J.D., Kelly, C.T. & Fogarty, W.M. Chemical modification of the α-amylase ofBacillus caldovelox with diethyl pyrocarbonate: Evidence for an essential histidine at the active site. Journal of Industrial Microbiology 9, 63–68 (1992). https://doi.org/10.1007/BF01576369
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01576369