Abstract
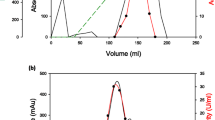
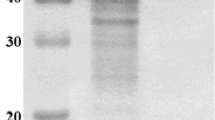
A color variant strain ofAureobasidium pullulans (NRRL Y-12974) produced amylase and α-glucosidase activities when grown at 28°C for 4 days in liquid culture on a wide variety of carbon sources such as starch, pullulan, glucose, maltose, cyclodextrins, sucrose, xylose, and xylan. An α-glucosidase was separated by Q-Sepharose adsorption from the cell-free culture broth and partially purified by hydroxylapatite and octyl-Sepharose chromatography. After ammonium sulfate treatment of the culture supernatant (obtained after Q-Sepharose adsorption), the amylase fraction was separated into three active fractions by hydroxylapatite column chromatography, which were identified as α-amylase, glucoamylase A, and glucoamylase B. The glucoamylase A was further purified by octyl-Sepharose column chromatography. The pH optima for the action of α-amylase, glucoamylase A, glucoamylase B, and α-glucosidase were 5.0, 4.5, 4.0–4.5, and 4.5, respectively. The α-amylase and glucoamylase B were fully stable at pH 3.0–6.0, glucoamylase A at pH 4.5–5.5, and α-glucosidase at pH 3.5–7.0 for 1 h at 50°C. The optimum temperatures for the action of these enzymes were 55°, 50°–60°, 65°, and 65°C, respectively. The α-amylase, glucoamylase A, and glucoamylase B were adsorbed onto raw corn starch and degraded it. Glucoamylase B readily cleaved pullulan. The α-glucosidase was not adsorbed onto raw starch and did not degrade it at all. It hydrolyzed both α-1,4 and α-1,6 linkages in oligosaccharides. All four enzymes did not require any metal ion for activity and were inhibited by cyclodextrins (α-and β-, 10mm).
Similar content being viewed by others
Literature Cited
Cotta MA (1988) Amylolytic activity of selected species of ruminal bacteria. Appl Environ Microbiol 54:772–776
De Mot R, Verachtert H (1985) Purification and characterization of extracellular amylolytic enzymes from the yeastFilobasidium capsuligenum. Appl Environ Microbiol 50:1474–1482
De Mot R, Verachtert H (1986a) Secretion of α-amylase and multiple forms of glucoamylase by the yeastTrichosporon pullulans. Can J Microbiol 32:47–51
De Mot R, Verachter H (1986b) Enhanced production of extracellular α-amylase and glucoamylase by amylolytic yeasts using β-cyclodextrin as carbon source. Appl Microbiol Biotechnol 24:459–463
De Mot R, Verachtert H (1987) Purification and characterization of extra-cellular α-amylase and glucoamylase from the yeastCandida antarctica CBS 6678. Eur J Biochem 164: 643–654
Deshpande, MS, Rale VB, Lynch, JM (1992)Aureobasidium pullulans in applied microbiology: a status report. Enzyme Microb Technol. 14:514–527
Federici F (1982) Extracellular enzymatic activities inAureobasidium pullulans. Mycologia 74:738–743
Federici F (1984) Glucoamylase production byAureobasidium pullulans. Mycologia 76:643–649
Federici F, D'Elia M (1983) Growth and amylolytic activity ofAureobasidium pullulans in starch-limited culture. Enzyme Microb Technol 5:225–226
Federici F, Petruccioli M, Miller MW (1990) Enhancement and stabilization of the production of glucoamylase by immobilized cells ofAureobasidium pullulans in a fluidized-bed reactor. Appl Microbiol Biotechnol 33:407–409
Federici RG, Federici F, Petruccioli M (1990) Continuous production of glucoamylase by immobilized growing cells ofAureobasidium pullulans. Biotechnol Lett 12:661–666
Hugget ASC, Nixon DA (1957) Glucose oxidase method for measurement of glucose. Biochem J 6:12–19
Kato K, Kuswanto K, Banno I, Harada T (1976) Identification ofEndomycopsis fibuligera isolated from Ragi in Indonesia and properties of its crystalline glucoamylase. J Ferment Technol 54:705–708
Leathers TD (1986) Color variants ofAureobasidium pullulans overproduce xylanase with extremely high specific activity. Appl Environ Microbiol 52:1026–1030
Leathers TD (1981) Host amylases and pullulan production. In: Kaplan TD (ed) Materials Biotech Symp Proc. Natick, Mass.: US Army Natick Research Development and Engineering Center, pp.175–185
Leathers TD, Detroy RW, Bothast RJ (1986) Induction and glucose repression of xylanase from a color variant strain ofAureobasidium pullulans. Biotech Lett 8:867–872
Leathers TD, Nofsinger GW, Kurtzman CP, Bothast RJ (1988) Pullulan production by color variant strains ofAureobasidium pullulans. J Ind Microbiol 3:231–239
Linardi VR, Machado KMG (1990) Production of amylases by yeasts. Can J Microbiol 36:751–753
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Ohno N, Ijuin T, Song S, Uchiyama S, Shinoyama H, Ando A, Fuji T (1992) Purification and properties of amylases extracellularly produced by an imperfect fungus,Fusidium sp. BX-1 in a glycerol medium. BioSci Biotech Biochem 56:465–471
Saha BC, Ueda S (1983) Alcoholic fermentation of raw sweet potato by a nonconventional method usingEndomycopsis fibuligera glucoamylase preparation. Biotech Bioeng 25: 1181–1186
Saha BC, Zeikus JG (1991) Characterization of thermostable α-glucosidase fromClostridium thermohydrosulfuricum 39E. Appl Microbiol Biotechnol 35:568–571
Saha BC, Mitsue T, Ueda S (1979) Glucoamylase produced by submerged culture ofAspergillus oryzae. Starch/Starke 31:307–314
Silman RW, Bryan WL, Leathers TD (1990) A comparison of polysaccharides from strains ofAureobasidium pullulans. FEMS Microbiol Lett 71:65–70
Simoes-Mendes B (1984) Purification and characterization of the extracellular amylases of the yeastSchwanniomyces alluvis. Can J Microbiol 30:1163–1170
Spencer-Martins I, van Uden N (1979) Extracellular amylolytic system of the yeastLipomyces kononenkoae. Eur J Appl Microbiol Biotechnol 6:241–250
Tsujisaka Y, Mitsuhashi M (1988) Manufacture of pullulan. In: The Amylase Research Society of Japan (ed) Handbook of amylases and related enzymes. New York: Pergamon Press, pp 221–223
Ueda S, Saha BC (1983) Behavior ofEndomycopsis fibuligera glucoamylase towards raw starch. Enzyme Microb Technol 5:196–198
Wilson JJ, Ingledew WM (1982) Isolation and characterization ofSchwanniomyces alluvius amylolytic enzymes. Appl Environ Microbiol 44:301–307
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saha, B.C., Silman, R.W. & Bothast, r.J. Amylolytic enzymes produced by a color variant strain ofAureobasidium pullulans . Current Microbiology 26, 267–273 (1993). https://doi.org/10.1007/BF01575916
Issue Date:
DOI: https://doi.org/10.1007/BF01575916