Abstract
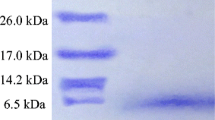
Piscicolin 61, a bacteriocin produced byCarnobacterium piscicola LV61, inhibits the growth of strains ofCarnobacterium, Lactobacillus, andEnterococcus. The bacteriocin was purified to homogeneity by ammonium sulfate precipitation and sequential hydrophobic interaction and reversed-phase chromatography. The N-terminal amino acid sequence of piscicolin 61 was determined by Edman degradation. The plasmid-located structural gene encoding piscicolin (psc61) was cloned and sequenced. It encoded a primary translation product of 71 amino acid residues, which is cleaved between amino acid residues 18 and 19 to yield the active bacteriocin. The calculatedM r from the deduced protein sequence, 5052.6, agreed with that obtained by mass spectrometry. Piscicolin 61 did not show any sequence similarities to other known bacteriocins. However, the leader sequence resembled those of the pediocin-like bacteriocins. Piscicolin 61 may be able to form amphiphilic helices and may thus act on the membrane of sensitive cells.
Similar content being viewed by others
Literature Cited
Ahn C, Stiles ME (1990a) Antibacterial activity of lactic acid bacteria isolated from vacuum-packaged meats. J Appl Bacteriol 69:302–310
Ahn C, Stiles ME (1990b) Plasmid-associated bacteriocin production by a strain ofCarnobacterium piscicola from meat. Appl Environ Microbiol 56:2503–2510
Ahn C, Stiles ME (1992) Mobilization and expression of bacteriocin plasmids fromCarnobacterium piscicola isolated from meats. J Appl Bacteriol 73:217–228
Aukrust T, Blom H (1992) Transformation ofLactobacillus strains used in meat and vegetable fermentations. Food Res Int 25:253–256
Axelsson L, Holck A, Birkeland S-E, Aukrust T, Blom H (1993) Cloning and nucleotide sequence of a gene fromLactobacillus sake Lb706 necessary for sakacin A production and immunity. Appl Environ Microbiol 59:2868–2875
Buchanan RL, Klawitter LA (1992) Effectiveness ofCarnobacterium piscicola LK5 for controlling the growth ofListeria monocytogenes scott A in refrigerated foods. J Food Safety 12:219–236
Cornwell GG, Sletten K, Johansson B, Westermark P (1988) Evidence that the amyloid fibril protein in senile systemic amyloidosis is derived from normal prealbumin. Biochem Biophys res Commun 154:648–653
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Hastings JW, Sailer M, Johnson K, Roy KL, Vederas JC, Stiles ME (1991) Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene fromLeuconostoc gelidum. J Bacteriol 173:7491–7500
Holck A, Axelsson L, Birkeland S-E, Aukrust T, Blom H (1992) Purification and amino acid sequence of sakacin A, a bacteriocin fromLactobacillus sake Lb706. J Gen Microbiol 138:2715–2720
Holck A, Axelsson L, Hühne K, Kröckel L (1994) Purification and cloning of sakacin 674, a bacteriocin fromLactobacillus sake Lb674. FEMS Microbiol Lett 115:143–150
Holo H, Nilssen Ø, Nes IF (1991) Lactococcin A, a new bacteriocin fromLactococcus lactis subsp.cremoris: isolation and characterization of the protein and its gene. J Bacteriol 173:3879–3887
Jardine I, Scanlan GF, Tsarbopoulos A, Liberato DJ (1988) Plasma desorption mass spectrometry of peptides adsorbed on nitrocellulose from a glutathione matrix. Anal Chem 60:1086–1088
Maftah A, Renault D, Vignoles C, Héchard Y, Bressollier P, Ratinaud MH, Cenatiempo Y, Julien R (1993) Membrane permeabilization ofListeria monocytogenes and mitochondria by the bacteriocin mesentericin Y105. J Bacteriol 175:3232–3235
Marugg JD, Gonzalez CF, Kunka BS, Ledeboer AM, Pucci MJ, Toonen MY, Walker SA, Zoetmulder LCM, Vandenbergh PA (1992) Cloning, expression, and nucleotide sequence of genes involved in production of pediocin PA-1, a bacteriocin fromPediococcus acidilactici PAC1.0. Appl Environ Microbiol 58:2360–2367
Muriana PM, Klaenhammer TR (1991) Cloning, phenotypic expression, and DNA sequence of the gene for lactacin F, an antimicrobial peptide produced byLactobacillus spp. J Bacteriol 173:1779–1788
Nettles CG, Barefoot SF (1993) Biochemical and genetic characteristics of bacteriocins of food-associated lactic acid bacteria. J Food Protection 56:338–356
Ojcius DM, Young JD-E (1991) Cytolytic pore-forming proteins and peptides: is there a common structural motif? Trends Biochem Sci 16:225–229
Rao JKM, Argos P (1986) A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta 869:197–214
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press
Sanger F, Nicklen S, Coulson R (1977) DNA-sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schillinger U, Holzapfel WH (1990) Antibacterial activity of carnobacteria. Food Microbiol 7:305–310
Schillinger U, Stiles ME, Holzapfel WH (1993) Bacteriocin production byCarnobacterium piscicola LV 61. Int J Food Microbiol 20:131–147
Stoddard GW, Petzel JP, van Belkum MJ, Kok J, McKay LL (1992) Molecular analysis of the lactococcin A gene cluster fromLactococcus lactis subsp.lactis biovar diacetylactis WM4. Appl Environ Microbiol 58:1952–1961
Stoffels G, Nissen-Meyer J, Gudmundsdottir A, Sletten K, Holo H, Nes IF (1992) Purification and characterization of a new bacteriocin isolated from aCarnobacterium sp. Appl Environ Microbiol 58:1417–1422
Tsarbopoulos A (1989) Plasma desorption mass spectrometry of natural and recombinant peptides and proteins. Peptide Res 2:258–266
van Belkum MJ, Hayema BJ, Jeeninga RE, Kok J, Venema G (1991a) Organization and nucleotide sequences of two lactococcal bacteriocin operons. Appl Environ Microbiol 57:492–498
van Belkum MJ, Kok J, Venema G, Holo H, Nes IF, Konings WN, Abee T (1991b) The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J Bacteriol 173:7934–7941
van Belkum MJ, Kok J, Venema G (1992) Cloning, sequencing and expression inEscherichia coli oflcnB, a third bacteriocin determinant from the lactococcal bacteriocin plasmid p9B4-6. Appl Environ Microbiol 58:572–577
von Wright A, Tynkkynen S, Suominen M (1987) Cloning of aStreptococcus lactis subsp.lactis chromosomal fragment associated with the ability to grow in milk. Appl Environ Microbiol 53:1584–1588
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holck, A.L., Axelsson, L. & Schillinger, U. Purification and cloning of piscicolin 61, a bacteriocin fromCarnobacterium piscicola LV61. Current Microbiology 29, 63–68 (1994). https://doi.org/10.1007/BF01575750
Issue Date:
DOI: https://doi.org/10.1007/BF01575750