Abstract
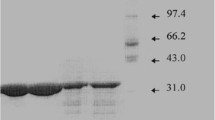
A method for the purification of enolase (EC 4.2.1.11) from an overproducing strain ofEscherichia coli JA 200 pLC 11–8 is described. The procedure included treatment of the crude sonic extract with protamine sulfate, followed by ammonium sulfate fractionation, hydrophobic interaction chromatography with phenyl Sepharose, HPLC ion exchange chromatography with a DuPont Sax column, and HPLC hydrophobic interaction chromatography with a Bio-Rad 5-PW column. The enzyme was purified to homogeneity as determined by silver staining of 10% sodium dodecylsulfate polyacrylamide gels. The native molecular weight ofE. coli enolase was found to be 92 kilodaltons and consisted of two subunits of identical molecular weight, 46 kilodaltons each. The isoelectric point was found to be 4.9.
Similar content being viewed by others
Literature Cited
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Entian KD, Neurer B, Mecke D (1983) Purification of three distinct isoenzymes from yeast. Anal Biochem 132:225–228
Frolich KU, Entian KD (1982) Regulation of gluconeogenesis in the yeastSaccharomyces cerevisiae. FEBS Lett 139:164–166
Garnak M, Reeves HC (1979) Purification and properties of phosphorylated isocitrate dehydrogenase ofEscherichia coli. J Biol Chem 254:7915–7920
Gottschalk G (1986) Bacterial metabolism, 2nd edn. New York, Heidelberg, Berlin: Springer-Verlag
Heydorn WE, Creed GJ, Marangos PJ Jacobowitz DM (1985) Identification of neuron-specific enolase and non-neuronal enolase in human and rat brain on two-dimensional polyacrylamide gels. J Neurochem 44:201–209
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophase T4. Nature (London) 227:680–685
Procelli Jr LJ, Small ED, Brewer JM (1978) Origin of multiple species of yeast enolase A on isoelectric focusing. Biochem Biophys Res Commun 82:316–321
Robertson EF, Reeves HC (1987) Purification and characterization of isocitrate lyase fromEscherichia coli. Curr Microbiol 14:347–350
Robertson EF, Dannelly HK, Malloy P, Reeves HC (1987) Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal Biochem 167:290–294
Spring TG, Wold F (1971) The purification and characterization ofEschernica coli enolase. J Biol Chem 246:6797–6802
Thomson J, Gerstenberger PD Goldberg DE, Gociar E, Orozco de Silva A, Fraenkel DG (1979) Col E1 hybrid plasmids forEscherichia coli genes of glycolysis and the hexose monophosphate shunt. J Bacteriol 137:502–506
Wold F (1971) Enolase. In: Boyer PD (ed) The Enzymes, vol 5. New York: Academic Press, pp 499–538
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dannelly, H.K., Reeves, H.C. Purification and characterization of enolase fromEscherichia coli . Current Microbiology 17, 265–268 (1988). https://doi.org/10.1007/BF01571326
Issue Date:
DOI: https://doi.org/10.1007/BF01571326