Abstract
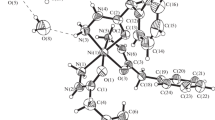
The crystal structure of tetrakisthiourea cobalt(II) nitrate monohydrate has been solved and refined by full-matrix least squares, including anisotropic temperature factors, to a finalR of 0.066. The structure is made up of discrete tetrakisthiourea Co(II) molecular ions and NO −3 ions bound together by hydrogen bonds with water molecules as well as ionic and van der Waals interactions. The local configuration of the cobalt(II) and its four bonded nearest sulfur neighbors is that of a distorted tetrahedron with an average Co-S distance of 2.30 Å. Three of the Co-S bonds appear to be made with sulfur sp2 orbitals and lone pairs whereas the remaining Co-S bond involves the S-C π molecular orbital as the electron donor. The thiourea groups appear to be normal. Tetrakisthiourea cobalt(II) nitrate monohydrate: orthorhombic,Pbca,a = 33.704(8),b = 11.734(2),c = 10.253(2) Å,Z = 8,D m = 1.68(2),D e = 1.66 g cm−3.
Similar content being viewed by others
References
Adams, D. M., and Cornell, J. B. (1967).J. Chem Soc. A 884.
Baggio, R. F., Manzanares, C. and Baggio, S. (1975).Acta Cryst. B 31, 2359.
Berta, D. A., Spofford, III, W. A., Boldrini, P., and Amma, E. L. (1970).Inorg. Chem. 9, 136–142.
Busing, W. R. & Levy, H. A. (1967).Acta Cryst. 22, 457.
Busing, W. R., Martin, K. O. and Levy, H. A. (1964). Oak Ridge National Laboratory report No. ORNL-TM-306, Oak Ridge, Tennessee.
Cotton, F. A., Faut, O. D., and Mague, J. T. (1964).Inorg. Chem. 3, 17.
Cromer, D. T. (1965).Acta Cryst. 18, 17.
Cromer, D. T., and Waber, J. T. (1965).Acta Cryst. 18, 104.
Enemark, J. H., and Lipscomb, W. N. (1965).Inorg. Chem. 4, 1729.
Gash, A. G., Griffith, E. H., Spofford, III, W. A., and Amma, E. L. (1973).J. Chem. Soc., Chem. Commun. 256.
Girling, R. L., Chatterjee, K. K., and Amma, E. L. (1973).Inorg. Chim. Acta 7, 557.
Griffith, E. A. H., Hunt, G. W., and Amma, E. L. (1976).J. Chem. Soc., Chem. Commun. 432.
Ibers, J. A., and Hamilton, W. C. (1964). Anomalous dispersion corrections made toF c ,as suggested, as suggested,Acta Cryst. 17, 781.
Kunchur, N. R., and Truter, M. R. (1958).J. Chem. Soc. 2551.
Knox, K. (1967). “Master Card Program for Picker Four-Angle Programmer,” p. 11, cited in Busing and Levy (1967).
Lopez-Castro, A., and Truter, M. R. (1963).J. Chem. Soc. 1309.
Nardelli, M., Gaspard, G., Battistini, G. G., and Domiano, P. (1966).Acta Cryst. 20, 349.
O'Connor, J. E., and Amma, E. L. (1969).Inorg. Chem. 8, 2367.
Spofford, III, W. A., and Amma, E. L. (1968).J. Chem. Soc., Chem. Commun. 405.
Spofford, III, W. A., and Amma, E. L. (1970).Acta Cryst. B 26, 1474.
Spofford, III, W. A., Boldrini, P., Amma, E. L., Carfagno, P., and Gentile, P. S. (1970).J. Chem. Soc., Chem. Commun. 40.
Stevenson, D. L., Magnuson, V. R., and Dahl, L. F. (1967).J. Am. Chem. Soc. 89, 3727.
Taylor, Jr., I. F., Weininger, M. S., and Amma, E. L. (1974).Inorg. Chem. 13, 2835.
Truter, M. R. (1967).Acta Cryst. 22, 556.
Vizzini, E. A., and Amma, E. L. (1966).J. Am. Chem. Soc. 88, 2872.
Vizzini, E. A., Taylor, Jr., I. F., and Amma, E. L. (1968).Inorg. Chem. 7, 1351.
Vranka, R. G., and Amma, E. L. (1966).J. Am. Chem. Soc. 88, 4270.
Weininger, M. S., O'Connor, J. E., and Amma, E. L. (1969).Inorg. Chem. 8, 424.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spofford, W.A., Amma, E.L. Crystal and molecular structure of tetrakisthiourea cobalt (II) nitrate monohydrate, C4H16CoN8S4 2+,2NO −3 ,H2O. Journal of Crystal and Molecular Structure 6, 235–258 (1976). https://doi.org/10.1007/BF01570985
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01570985