Abstract
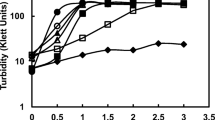
The marine non-heterocystous, filamentous cyanobacteriumPhormidium valderianum BDU 30501, which failed to grow in the absence of combined nitrogen in the medium, grew when supplemented with ampicillin. Growth in terms of both protein as well as chlorophyll was proportional to the concentration of ampicillin up to 2000 μg ml−1. With the hydroxylamine assay, the organism was found to produce β-lactamase extracellularly with activity reaching a maximum within 6 h of incubation. The maximum production and activity of the enzyme in the culture filtrate was at pH 7 at a concentration of 750 μg ml−1 ampicillin and at 25°C. The crude enzyme was thermolabile, because temperatures above 0°C on storage resulted in the loss of activity within hours. The enzyme could be induced both by ampicillin as well as benzyl penicillin, but ampicillin proved to be a more suitable substrate both in terms of induction as well as activity. This is the first report of an antibiotic serving as a nitrogen source for an organism.
Similar content being viewed by others
Literature Cited
Ayliffa GAJ (1963) Ampicillin inactivation and sensitivity of coliform bacilli. J Gen Microbiol 30:339.
Citri N, Pollock MR (1966) The biochemistry and function of β-lactamase (penicillinase) In: Nord FF (ed) Advances in enzymology, Vol. 28. New York: Inter Science Publishers, pp. 287–323
Hamilton-Miller JMT, Brumfitt W, Smith GN, Davis AJ (1968) Testing, reporting and prescribing antibiotics for urinary infection. J Antimicrob Chemother 10:7–9
Holt RJ, Stewart GT (1963) Techniques for the rapid and sensitive detection of penicillanase. J Clin Pathol 16:233
Holt RJ, Stewart GT (1964) Production of amidase and β-lactamase by bacteria. J Gen Microbiol 36:203–213
Kumar HD (1968) Inhibitory action of the antibiotics mitomycin C and neomycin on the blue-green algaAnacystis nidulans. Flora Jena 159:437–444
Kushner DJ, Breuil C (1977) Penicillanase (β-lactamase) formation by blue-green algae. Arch Microbiol 112:219–223
Lowrey OH, Rosenburgh NJ, Farr AL, Randali RJ (1951) Protein measurement with the Folin-phenol reagent. J Biol Chem 193:265–275
MacKinney G (1941) Absorption of light by chlorophyll solution. J Biol Chem 140:314–322
Maharana RK, Pradhan JM, Padhi S (1986) Effect of amoxycillin and cephaloxin on growth and nitrogen fixation ofCalothrix marchica. IBC 3:45–47
Melling J, Scott GK (1972) Preparation of gram quantities of a purified R factor mediated penicillanase fromEscherichia coli strain W 3310. Biochem J 130:55–67
Novick RP (1964) Analysis by transduction of mutation affecting penicillanase formation inStaphylococci aureus. J Gen Microbiol 33:121–136
Prabaharan D (1989) Isolation and partial characterization of a marine cyanobacterium for biotechnological purposes. J Ind Bot Soc 67(Suppl.):10–11
Reynaud PA, Franche C (1980) Isolation and characterization of non-heterocystous tropical cyanobacteria growing on nitrogen free medium. MIRCEN J 2:427–443
Richmond MH (1975) β-lactamase (Staphylococcus aureus) Methods Enzymol 43:664–672
Rippka R, Waterbury JB, Stanier RY (1981) Isolation and purification of cyanobacteria: some general principles. In: Starr MP, Stolp H, Truper HG, Balows A, Schlegel HG (eds) The Prokaryotes, a handbook on habitats, isolation and identification of bacteria. New York: Springer-Verlag, pp 212–223
Sargent MG (1968) Rapid fixed-time assay for penicillinase. J Bacteriol 1493–1494
Srivastava BS (1969) Ultra-violet induced mutation to growth factor requirement and penicillin resistance in a blue-green alga. Arch Microbiol 66:234–238
Thatcher DR (1975a) β-lactamase (Bacillus cereus), In: Methods Enzymol 43:640–652
Thatcher DR (1975b) β-lactamase (Bacillus licheniformis), In: Methods Enzymol 43:653–663
Vance BD (1966) Sensitivity ofMicrocystis aeruginosa and other blue-green algae and associated bacteria to selected antibiotics. J Phycol 2:125–128
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prabaharan, D., Sumathi, M. & Subramanian, G. Ability to use ampicillin as a nitrogen source by the marine cyanobacteriumPhormidium valderianum BDU 30501. Current Microbiology 28, 315–320 (1994). https://doi.org/10.1007/BF01570194
Issue Date:
DOI: https://doi.org/10.1007/BF01570194