Abstract
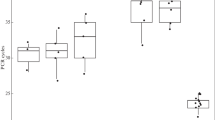
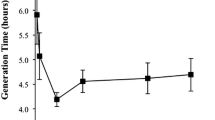
The lethality and mutagenicity in ethyl methanesulfonate (EMS)-treated cells of five archaebacterial strains belonging to each of the three described genera of non-alkaliphilic halobacteria were investigated. In order to test the efficiency of the mutagenesis under a variety of experimental conditions, we chose the fast-growing halobacteriumHaloferax mediterranei as a model strain. A strong induced mutagenicity was found, since the spontaneous mutation rate (expressed as the rate of resistance to the antibiotic josamycin) increased up to 500-fold after mutagen exposure. The mutagenesis was also successfully used in obtaining auxotrophic mutants. Although a heterogeneous response to the induced effects caused after EMS exposure was detected for the other halophilic archaebacteria tested, a clear, efficient mutagenicity ofHalobacterium halobium andHaloferax gibbonsii was observed; auxotrophic mutants of this halobacterium were also produced. Optimal experimental conditions for EMS mutagenesis of some halobacteria were determined.
Similar content being viewed by others
Literature Cited
Antón J, Meseguer I, Rodriguez-Valera F (1988) Production of an extracellular polysaccharide byHaloferax mediterranei. Appl Environ Microbiol 54:2381–2386
Bock A, Kandler O (1985) Antibiotic sensitivity of archaebacteria In: Woese CR, Wolfe RS (eds) The bacteria, vol. 8, New York: Academic Press, pp 525–544
Bonelo G, Megías M, Ventosa A, Nieto JJ, Ruiz-Berraquero F (1984) Lethality and mutagneicity inHalobacterium mediterranei caused by N-methyl-N′-nitro-N-nitrosoguanidine. Curr Microbiol 11:165–170
Bowring SM, Morris JG (1985) Mutagenesis ofClostridium acetobutylicum. J Appl Bacteriol 58:577–584
Cline SW, Doolittle WF (1987) Efficient transfection of the archaebacteriumHalobacterium halobium. J Bacteriol 169:1341–1344
Cline, SW, Lam WL, Charlebosis RL, Schalwkyk LC, Doolittle WF (1989) Transformation methods for halophilic archaebacteria. Can J Microbiol 35:148–152
Doolittle WF (1985) Genome structure in archaebacteria. In: Woese CR, Wolfe RS (eds) The bacteria, vol. 8. New York: Academic Press, pp 545–560
Fitt PS, Sharma N, Barna N (1989) Studies of the effects of liquid holding on viability and mutation frequency in N-methyl-N′-nitro-N-nitrosoguanidine-treated halophiles. Curr Microbiol 18:87–91
Gomori G (1955) Preparation of buffers for use in enzyme studies. In: Methods Enzymol 1: 138–146
Juez G, Rodriguez-Valera F, Mojica FJM, (1990) Evidence for salt-associated restriction pattern modifications in the archaebacteriumHaloferax mediterranei. J Bacteriol 172:7278–7281
Kushner DJ (1985) The Halobacteriaceae. In: Woese CR, Wolfe RS (eds) The bacteria, vol. 8. New York: Academic Press, pp 171–214
Mevarech M, Werczberger R (1985) Genetic transfer inHalobacterium volcanii. J Bacteriol 162:461–462
Oesterhelt D, Kripphal G (1983) Phototrophic growth of halobacteria and its use for isolation of photosynthetically deficient mutants. Ann Microbiol (Inst. Pasteur) 134B:137–150
Pfeiffer F (1988) Genetics of halobacteria. In: Rodriguez-Valera F (ed) Halophilic bacteria, vol. 2. Boca Raton: CRC Press, pp 105–133
Rosenshine I, Zusman T, Werczberger R, Mevarech M (1989) Amplification of specific DNA sequences correlates with resistance of the archaebacteriumHalobacterium volcanii to the dihydrofolate reductase inhibitors trimethoprim and methotrexate. Mol Gen Genet 298:518–522
Sega GA (1984) A review of the genetic effects of ethyl methanesulfonate. Mutat Res 134:113–142
Sweet DM, Moseley BEB (1976) The resistance ofMicrococcus radiodurans to killing and mutation by agents which damage DNA. Mutat Res 34:175–186
Woese CR, Fox GE (1977) Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci USA 74:5088–5090
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nieto, J.J., Fernández-Castillo, R., Megías, M. et al. Ethyl methanesulfonate mutagenesis in extremely halophilic archaebacteria: Isolation of auxotrophic mutants ofHaloferax mediterranei andHaloferax gibbonsii . Current Microbiology 24, 41–47 (1992). https://doi.org/10.1007/BF01570098
Issue Date:
DOI: https://doi.org/10.1007/BF01570098