Abstract
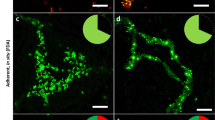
A lectin-biotin assay was developed for use in the specific detection of slime produced byStaphylococcus epidermidis RP62A and M187sp11 grown in a chemically defined medium. Mature biofilm was formed on polyvinylchloride (PVC) disks using a combined chemostat-modified Robbins device (MRD) model system. Specimens fixedin situ were: 1) stained with ruthenium red; 2) reacted overnight with biotin-labeled lectins (WGA, succinyl-WGA, Con A, or APA) followed by treatment with gold-labeled extravidin; or 3) reacted with antibodies againstS. epidermidis RP62A capsular polysaccharide/adhesin (PS/A) using an immunogold procedure. WGA and succinyl-WGA (S-WGA), which specifically bindN-acetylglucosamine, were shown by TEM to react only with slime, both cell-associated and exocellular. In contrast, Con A, APA and anti-PS/A reacted with the bacterial cell surface but did not react with slime. These results indicate the usefulness of WGA lectin as a specific marker for detection of the presence and distribution of slime matrix material inS. epidermidis biofilm.
Similar content being viewed by others
References
Arvaniti A, NK Karamanos, G Dimitracopoulos and ED Anastassiou. 1994. Isolation and characterization of a novel 20-kDa sulfated polysaccharide from the extracellular slime layer ofStaphylococcus epidermidis. Arch Biochem Biophys 308: 432–438.
Barth E, QM Myrvik, W Wagner and G Gristina. 1989.In vitro andin vivo comparative colonization ofStaphylococcus aureus andStaphylococcus epidermidis on orthopaedic implant materials. Biomaterials 10: 325–328.
Bayston R and SR Penny. 1972. Excessive production of mucoid substance inStaphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol 14 (supplement 27): 25–28.
Christensen GD, WA Simpson, AL Bison and EH Beachey. 1982. Adherence of slime-producing strains ofStaphylococcus epidermidis to smooth surfaces. Infect Immun 37: 318–326.
Christensen GD, WA Simpson, JJ Younger, LM Baddour, FF Barrett, DM Melton and EH Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22: 996–1006.
Christensen GD, TP Barker, TP Mawhinney, LM Baddour and WA Simpson. 1990. Identification of an antigenic marker of slime production forStaphylococcus epidermidis. Infect Immun 58: 2906–2911.
Costerton JW, K-J Cheng, GG Geesey, TI Ladd, JC Nickel, M Dasgupta and TJ Marrie. 1987. Bacterial biofilms in nature and disease. Ann Rev Microbiol 41: 435–464.
Dankert J, AH Hogt and J Feijen. 1986. Biomedical polymers: bacterial adhesion, colonization, and infection. CRC Crit Rev Biocompat 2: 219–301.
Davidson SK, KF Keller and RJ Doyle. 1982. Differentiation of coagulase-positive and coagulase-negative staphylococci by lectins and plant agglutinins. J Clin Microbiol 15: 547–553.
Doyle RJ. 1994. Introduction to lectins and their interactions with microorganisms. In. Lectin-Microorganism Interactions (Doyle RJ and M Slifkin, eds), pp 1–65, Marcel Dekker, New York.
Drewry DT, L Galbraith, BJ Wilkinson and SG Wilkinson. 1990. Staphylococcal slime: a cautionary tale. J Clin Microbiol 28: 1292–1296.
Estrada JC, NT Brinn and EH Bossen. 1985. A rapid method of staining ultrathin sections for surgical pathology TEM with the use of the microwave oven. Am J Clin Path 83: 639–641.
Hayat MA. 1970. Staining. In: Principles and Techniques of Electron Microscopy, Biological Applications 1, pp 262–264 and 345, Van Nostrand Reinhold Co, New York.
Hussain M, JGM Hastings and PJ White. 1991. Isolation and composition of the extracellular slime made by coagulase-negative staphylococci in a chemically defined medium. J Infect Dis 163: 534–541.
Ludwicka A, G Uhlenbruck, G Peters, PN Send, ED Gray, J Jeljaszewicz and G Pulverer. 1984. Investigation on extracellular slime substance produced byStaphylococcus epidermidis. Zentralblatt fuer Bakteriologie Hyg A 258: 256–267.
Luft JH. 1971. Ruthenium red and violet I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec 171: 347–368.
Milligan TW, TI Doran, DC Straus and SJ Mattingly. 1978. Studies on the growth and amino acid requirements of group B streptococci. J Clin Microbiol 7: 28–33.
Muller E, J Hubner, N Gutierrez, S Takeda, DA Goldmann and GB Pier. 1993. Isolation and characterization of transposon mutants ofStaphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect Immun 61: 551–558.
Muller E, S Takeda, H Shiro, D Goldmann and GB Pier. 1993. Occurrence of capsular polysaccharide/adhesin among clinical isolates of coagulase-negative staphylococci. J Infect Dis 168: 1211–1218.
Nickel JC, I Ruseska, JB Wright and JW Costerton. 1985. Tobramycin resistance ofPseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents and Chemother 27: 619–623.
Peters G, R Locci and G Pulverer. 1982. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis 146: 479–482.
Peters G, F Schumacher-Perdreau, B Jansen, M Bey and G Pulverer. 1987. Biology ofS. epidermidis extracellular slime. Zbl Bakt Suppl 16: 15–31.
Reeder WJ and RD Ekstedt. 1971. Study of the interaction of concanavalin A with staphylococcal teichoic acids. J Immunol 106: 334–340.
Sanford BA, S Mattingly, V Thomas, H Rawls, B Norling and M Ramsay. 1993.Staphylococcus epidermidis adhesion and biofilm formation using a combined chemostat-modified Robbins device model system. IEEE Proceedings of the Twelfth Southern Biomedical Engineering Conference, pp 254–256.
Slusher MM, QN Byrvik, JC Lewis and AG Gristina. 1987. Extendedwear lenses, biofilm, and bacterial adhesion. Arch Ophthalmol 105: 110–115.
Tojo M, N Yamashita, DA Goldmann and GB Pier. 1988. Isolation and characterization of a capsular polysaccharide adhesin fromStaphylococcus epidermidis. J Infect Dis 157: 713–722.
Wilcox MH, M Hussain, MK Faulkner, PJ White and RC Spencer. 1991. Slime production and adherence by coagulase-negative staphylococci. J Hosp Infect 18: 327–332.
Wilkinson JF. 1958. The extracellular polysaccharides of bacteria. Bacteriol Rev 22: 46–73.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sanford, B.A., Thomas, V.L., Mattingly, S.J. et al. Lectin-biotin assay for slime present inin situ biofilm produced byStaphylococcus epidermidis using transmission electron microscopy (TEM). Journal of Industrial Microbiology 15, 156–161 (1995). https://doi.org/10.1007/BF01569820
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01569820