Summary
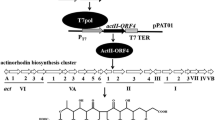
Streptomyces clavuligerus isopenicillin N synthase (IPNS) gene expression was achieved inEscherichia coli by the construction of two-cistron expression systems formed in the high copy number plasmid vector pUC119. These two-cistron constructions were composed of the IPNS gene and its flanking sequences which encoded an upstream open reading frame (ORF), the IPNS ribosome binding site and a putative transcription terminator. NoE. coli- likeStreptomyces promoter motif was present upstream of the IPNS gene therefore transcriptional regulation of the two-cistron system was provided by thelac promoter of pUC119. Enzymatically active IPNS was detected inE. coli cells harboring the recombinant plasmids thereby providing evidence for the activity of the IPNS ORF and for the feasibility of production ofS. clavuligerus IPNS inE. coli. These results indicate that simple two-cistron constructions involving foreign gene flanking sequences may be used to express foreign proteins inE. coli.
Similar content being viewed by others
References
Bagdasarian, M., R. Lurz, B. Rückert, F.C.H. Franklin, M.M. Bagdasarian, J. Frey and K.N. Timmis. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning inPseudomones. Gene 16: 237–247.
Baldwin, J.E. and E. Abraham. 1988. Biosynthesis of penicillins and cephalosporins. Nat. Prod. Rep. 5: 129–145.
Baldwin, J.E., S.J. Killin, A.J. Pratt, J.D. Sutherland, N.J. Turner, M.J.C. Crabbe, E.P. Abraham and A.C. Willis. 1987. Purification and characterization of cloned isopenicillinN synthetase. J. Antibiot. 40: 652–659.
Bibb, M.J. and S. Cohen. 1982. Gene expression inStreptomyces: construction and application of promoter-probe plasmid vectors inStreptomyces lividans. Mol. Gen. Genet. 187: 265–277.
Bibb, M.J., P.R. Findlay and M.W. Johnson. 1984. The relationship between base composition and codon usage in bacterial genes and its use in the simple and reliable identification of protein coding sequences. Gene 30: 157–166.
Blackshear, P.J. 1984. Systems for polyacrylamide gel electrophoresis. Methods Enzymol. 104: 237–255.
Bradford, M. 1976. A rapid, sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Buttner, M.J., A.M. Smith and M.J. Bibb. 1988. At least three different RNA polymerase holoenzymes direct transcription of the agarase gene (dagA) ofStreptomyces coelicolor A3(2). Cell 52: 599–607.
Carr, L.G., P.L. Skatrud, M.E. Scheetz II, S.W. Queener and T.D. Ingolia. 1986. Cloning and expression of the isopenicillinN synthetase gene fromPenicillium chrysogenum Gene 48: 257–266.
Casadaban, M.J. and S.N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning inEscherichia coli. J. Mol. Biol. 138: 179–207.
Chen, H.-Z. and G. Zubay. 1983. Prokaryotic coupled transcription-translation. Methods Enzymol. 101: 674–690.
Demain, A.L. 1986. A new twist in beta-lactam biosynthesis. Bio/Technol. 4:18.
Deng, Z., T. Kieser and D.A. Hopwood. 1987. Activity of aStreptomyces transcriptional terminator inEscherichia coli. Nucleic Acids Res. 15: 2665–2675.
Duffaud, G.D., P.E. March and M. Inouye. 1987. Expression and secretion of foreign proteins inEscherichia coli. Methods Enzymol. 153: 492–516.
Fairbanks, G., T.L. Steck and D.F.H. Wallach. 1971. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10: 2606–2617.
Gergens, J.P., R.H. Stern and P.C. Wensink. 1979. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 7: 2115–2136.
Glick, B.R. and G.K. Whitney. 1987. Factors affecting the expression of foreign proteins inEscherichia coli. J. Ind. Microbiol. 1: 277–282.
Gold, L. 1988. Posttranscriptional regulatory mechanisms inEscherichia coli. Ann. Rev. Biochem. 57: 199–233.
Hu, N. and J. Messing. 1982. The making of strand-specific M13 probes. Gene 17: 271–277.
Jensen, S.E. 1986. Biosynthesis of cephalosporins. CRC Crit. Rev. Biotechnol. 3: 277–301.
Jensen, S.E., B.K. Leskiw, L.C. Vining, Y. Aharonowitz, D.W.S. Westlake and S. Wolfe. 1986. Purification of isopenicillinN synthetase fromStreptomyces clavuligerus. Can J. Microbiol. 32: 953–958.
Jensen, S.E., D.W.S. Westlake and S. Wolfe. 1982. Cyclization of δ-(l-α-aminoadipyl)-l-cysteinyl-d-valine to penicillins by cell-free extracts ofStreptomyces clavuligerus. J. Antibiot. 35: 483–490.
Jensen, S.E., D.W.S. Westlake and S. Wolfe. 1982. High performance liquid chromatographic assay of cyclization activity in cell-free systems fromStreptomyces clavuligerus. J. Antibiot. 35: 1026–1032.
Leskiw, B.K., Y. Aharonowitz, M. Mevarrech, S. Wolfe, L.C. Vining, D.W.S. Westlake and S.E. Jensen. 1988. Cloning and nucleotide sequence determination of the isopenicillinN synthetase gene fromStreptomyces clavuligerus. Gene 62: 187–196.
Maniatis, T., E.F. Fritsch and J. Sambrook. 1982. Molecular Cloning: A Laboratory Manual. Cold Springer Harbor Laboratory, Cold Spring Harbor, New York.
Martin, R. 1987. Overcoming DNA sequencing artifacts: stops and compressions. BRL Focus 9: 8–10.
Morrison, D.A. 1979. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 68: 326–331.
Pulido, D. and A. Jimenez. 1987. Optimization of gene expression inStreptomyces lividans by a transcription terminator. Nucleic Acids Res. 15: 4227–4240.
Ramon, D., L. Carramolino, C. Patino, F. Sanchez and M.A. Penalva. 1987. Cloning and characterization of the isopenicillin N synthetase gene mediating the formation of the β-lactam ring inAspergillus nidulans. Gene 57: 171–181.
Rigaud, G., T. Grange and R. Pictet. 1987. The use of NaOH as transfer solution of DNA onto nylon membrane decreases hybridization efficiency. Nucleic Acids Res. 15: 857.
Samson, S.M., R. Belagaje, D.T. Blankenship, J.L. Chapman, D. Perry, P.L. Skatrud, R.M. VanFrank, E.P. Abraham, J.E. Baldwin, S.W. Queener and T.D. Ingolia. 1985. Isolation, sequence determination and expression inE. coli of the isopenicillinN synthetase gene fromCephalosporium acremonium. Nature 318: 191–194.
Samson, S.M., J.L. Chapman, R. Belagaje, S.W. Queener and T.D. Ingolia. 1987. Analysis of the role of cysteine residues in isopenicillinN synthetase activity by site directed mutagenesis. Proc. Natl. Acad. Sci. U.S.A. 84: 5705–5709.
Samson, S.M., J.E. Dotzlaf, M.L. Slisz, G.W. Becker, R.M. VanFrank, L.E. Veal, W.-K. Yeh, J.R. Miller, S.W. Queener and T.D. Ingolia. 1987. Cloning and expression of the fungal expandase/hydroxylase gene involved in cephalosporin biosynthesis. Bio/Technol. 5: 1207–1214.
Sanger, F. and A.R. Coulson. 1978. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 87: 107–110.
Sanger, F., S. Nicklen and A.R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U.S.A. 74: 5463–5467.
Schoner, B.E., R.M. Belagaje and R.F. Schoner. 1986. Translation of a synthetic two-cistron mRNA inEscherichia coli. Proc. Natl. Acad. Sci. U.S.A. 83: 8506–8510.
Shiffman, D., M. Mevarech, S.E. Jensen, G. Cohen and Y. Aharonowitz. 1988. Cloning and comparative sequence analysis of the gene coding for isopenicillinN synthase inStreptomyces. Mol. Gen. Genet. 214: 562–573.
Smith, H.O. and M.L. Birnsteil. 1976. A simple method of DNA restriction mapping. Nucleic Acids Res. 3: 2387–2398.
Stanssens, P., E. Remaut and W. Fiers. 1986. Inefficient translation initiation causes premature transcription termination in thelacZ gene. Cell 44: 711–718.
Uchiyama, H. and B. Weisblum. 1985.N-Methyl transferase ofStreptomyces erythraeus that confers resistance to the macrolide-lincosamide-streptogramin B antibiotics: amino acid sequence and its homology to cognate R-factor enzymes from pathogenic bacilli and cocci. Gene 38: 103–110.
Vieira, J. and J. Messing. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153: 3–11.
Weigel, B.J., S.G. Burgett, V.J. Chen, P.L. Skatrud, C.A. Frolik, S.W. Queener and T.D. Ingolia. 1988. Cloning and expression inEscherichia coli of isopenicillinN synthetase genes fromStreptomyces lipmanii andAspergillus nidulans. J. Bacteriol. 170: 3817–3826.
Westpheling, J., M. Ranes and R. Losick. 1985. RNA polymerase heterogeniety inStreptomyces coelicolor. Nature 313: 22–27.
Wolfe, S., A.L. Demain, S.E. Jensen and D.W.S. Westlake. 1984. Enzymatic approach to syntheses of unnatural betalactams. Science 226: 1386–1392.
Yanisch-Perron, C., J. Vieira and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33: 103–119.
Zuker, M. and P. Stiegler. 1981. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 9: 133–148.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doran, J.L., Leskiw, B.K., Petrich, A.K. et al. Production ofStreptomyces clavuligerus isopenicillin N synthase inEscherichia coli using two-cistron expression systems. Journal of Industrial Microbiology 5, 197–206 (1990). https://doi.org/10.1007/BF01569677
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01569677