Summary
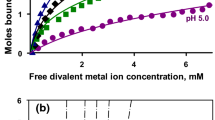
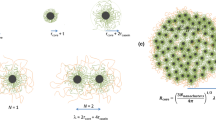
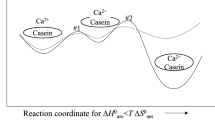
Molecular biology holds the promise of new tools for the food industry which include proteins with tailor-made functionality. Without a fundamental knowledge of the molecular bases of these properties, implementation will be strictly empirical. For example, the phenomena of salt-induced precipitation of proteins (salting-out) and their resolubilization (salting-in) has heretofore been discussed only qualitatively. A quantitative method, using Wyman's theory of thermodynamic linkage, has been developed and tested on the calcium-induced solubility profiles of the major milk proteins, the caseins. Salting-out was described by a salt-binding constant,k 1, andn, the number of moles of salt bound; salting-in was described by the corresponding termsk 2 andm. The magnitude of these parameters indicated involvement of protein phosphate groups in binding and precipitation, but enzymatic dephosphorylation showed significant increases ink 1 andk 2 indicating involvement of carboxylate groups as well. Studies on two genetic variants of αs1-casein indicated the importance of a hydrophobically stabilized intramolecular ion pair in the functionality of the protein. These studies have led to a fuller understanding of the molecular basis for the solubility behavior of caseins and have laid the groundwork for future computer simulation of food protein functionality.
Similar content being viewed by others
References
Bingham, E.W., H.M. Farrell, Jr. and R.J. Carroll. 1972. Properties of dephosphorylated αs1-casein. Precipitation by calcium ions and micelle formation. Biochemistry 11: 2450–2454.
Bingham, E.W., H.M. Farrell, Jr. and K.J. Dahl. 1976. Removal of phosphate groups from casein with potato acid phosphatase. Biochim. Biophys. Acta 429:448–460.
Creamer, L.K. and D.F. Waugh. 1966. Calcium binding and precipitate solvation of casein. J. Dairy Sci. 49:706 (Abstr.).
Creamer, L.K., T. Richardson and D.A.D. Parry. 1981. Secondary structure of bovine αs1- and β-caseins. Arch. Biochem. Biophys. 211: 689–702.
Dickson, I.R. and J.D. Perkins. 1971. Studies on the interactions between purified bovine caseins and alkaline earth metal ions. Biochem. J. 124: 235–240.
Eigel, W.N., J.E. Butler, C.A. Ernstrom, H.M. Farrell, Jr., V.R. Harwalkar, R. Jenness and R. McL. Whitney. 1984. Nomenclature of the proteins of milk: 5th revision. J. Dairy Sci. 67: 1599–1631.
Farrell, H.M., Jr. and M.P. Thompson. 1988. The caseins of milk as calcium binding proteins In: Calcium Binding Proteins (Thompson, M.P., ed.), CRC Press, Boca Raton, FL.
Linde, A. 1982. Calcium metabolism in dentinogenesis In: The Role of Calcium in Biological Systems, Vol. III (Anghileri, L.J. and A.M. Tuffet-Anghileri, eds.). CRC Press, Boca Raton, FL.
Linderstrom-Lang, K. 1929. On the heterogeneity of bovine casein. C. R. Trav. Lab. Carlesberg 17: 1–116.
Noble, R.W. and D.F. Waugh. 1965. Casein micelles, formation and structure I. J. Am. Chem. Soc. 87: 2236–2245.
Oppenheim, F.G., G.D. Offner and R.F. Troxler. 1982. Phosphoproteins in parotid saliva from the subhuman primateM. fascicularis. J. Biol. Chem. 257: 9271–9282.
Schmidt, D.G. 1984. Association of caseins and casein micelle structure. In: Developments in Dairy Chemistry (Fox, P.F. ed.), Applied Science Publications Ltd., London.
Stewart, A. F., I.M. Willis and A.G. MacKinlay. 1984. Nucleotide sequencesof bovine αs1- and k-caseins cDNA's. Nucleic Acids Res. 12: 3895–3907.
Tanford, C. 1967. Physical Chemistry of Macromolecules. John Wiley & Sons, New York.
Thompson, M.P., W.G. Gordon, R.T. Boswell and H.M. Farrell, Jr. 1969. Solubility solvation and stabilization of αs1- and β-caseins. J. Dairy Sci. 52: 1166–1173.
Waugh, D.F. and R.W. Noble. 1965. Casein micelles, formation and structure II. J. Am. Chem. Soc. 87: 2246–2257.
Waugh, D.F., C.W. Slattery and L.K. Creamer. 1971. Binding of calcium to caseins. Biochemistry 10: 817–823.
Wyman, J., Jr. 1964. Adv. Protein Chem. 19: 224–285.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Farrell, H.M., Kumosinski, T.F. Modeling of calcium-induced solubility profiles of casein for biotechnology: Influence of primary structure and posttranslational modification. Journal of Industrial Microbiology 3, 61–71 (1988). https://doi.org/10.1007/BF01569548
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01569548