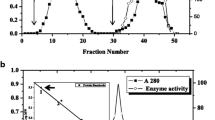
Abstract
The molecular properties, such as molecular weight, N-and C-terminal amino acids, amino acid composition, and circular dichroism, of 1,2-α-mannosidase isolated from the culture filtrate ofAspergillus saitoi were determined.
The enzyme had aK m of 0.67 mM andk cat of 1.27/s with mannobiose at pH 50.0 and 30°C. The anomeric configuration of the reaction products of the enzyme was examined by studying the α-anomer. A single Manαl→2Man linkage in intact Taka-amylase A fromAspergillus oryzae was hydrolyzed, producing free mannose.
Similar content being viewed by others
Literature Cited
Beychoks S (1966) Cirular dichroism of biological macromolecules. Science 154:1288–1299
Canfield RE, Anfinsen CB (1963) Chromatography of pepsin and chymotrypsin digests of egg white lysozyme on phosphocellulose. J Biol Chem 238:2684–2690
Gray WR (1972) End-group analysis using dansyl chloride. Methods Enzymol 25:121–138
Hoare DG, Koshland DE Jr (1968) A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem 242:2447–2453
Ichishima E, Arai M, Shigematsu Y, Kumagai H, Sumida-Tanaka R (1981) Purification of an acidic-α-d-mannosidase fromAspergillus saitoi and specific cleavage of 1,2α-d-mannosidic linkage in yeast manan. Biochim Biophys Acta 658:45–53
Ichishima E, Emi M, Majima E, Mayumi Y, Kumagai H, Hayashi K, Tomoda K (1982) Initial sites of insulin cleavage and sterospecificity of carboxyl proteinase fromAspergillus sojae andPycnoporus coccineus. Biochim Biophys Acta 700:247–253
Ichishima E, Yoshimura Y, Tomoda K (1983) Acid carboxypeptidase from wood-deteriorating Basidiomycete,Pycnoporus sanguineus. Phytochemistry 22:825–829
Inokuchi N, Iwana M, Takahashi T, Irie M (1982) Modification of a glucoamylase fromAspergillus saitoi with 1-cyclohexyl-3-(2-morpholinyl)-(4)-ethyl carbodiimide. J Biochem 91:125–133
Kocourek J, Ballou CE (1969) Method for fingerprinting yeast cell wall mannans. J Bacteriol 100:1175–1181
Minobe S, Nakajima H, Itoh N, Funakoshi I, Yamashina I (1979) Structure of a major oligosaccharide of Taka-amylase A. J Biochem 86:1851–1854
Riem J, Scheraga HA (1966) Structural studies of ribonuclease. XXI. Reaction between ribonuclease and a water-soluble carbodiimide. Biochemistry 5:99–115
Tai T, Yamashita K, Ogata-Arakawa M, Koide N, Muramatsu T, Iwashita S, Inoue Y, Kobata A (1975) Structural studies of two ovalbumin in glycopeptides in relation to the endo ß-acetylglucosamidase specificity. J Biol Chem 250:8569–8575
Tanaka N, Takauchi M, Ichishima E (1977) Purification of an acidic propteinase fromAspergillus saitoi and determination of peptide bond specificity. Biochim Biophys Acta 485:406–416
Toda H, Akabori S (1963) Chromatography of Taka-amylase A on diethylaminoethyl-cellulose column. J Biochem 53:102–110
Weber K, Osborn M (1969) The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem 244:4406–4412
Yamaguchi H, Ikenaka T, Matshushima Y (1970) The complete sequence of a glycopeptide obtained from Taka-amyplase A. J Biochem 70:587–594
Yamamoto K, Hitomi J, Kobataki K, Yamaguchi H (1982) Purification and characterization of 1,2-α-mannosidase ofAspergillus oryzae. J. Biochem 91:1971–1979
Yamashita K, Ichishima E, Arai M, Kobata A (1980) An α-mannosidase purified fromAspergillus saitoi is specific for α1,2 linkages. Biochem Biophys Res Commun 96:1335–1342
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shigematsu, Y., Tsukahara, K., Tanaka, T. et al. Molecular and enzymatic properties of 1,2-α-d-mannosidase fromAspergillus saitoi . Current Microbiology 13, 43–46 (1986). https://doi.org/10.1007/BF01568158
Issue Date:
DOI: https://doi.org/10.1007/BF01568158