Abstract
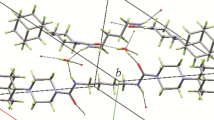
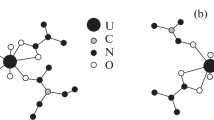
Urea (U) and salicylic acid (SA) crystallize from aqueous solution as a 1∶1 adduct whose structure shows them to be linked via several weak and one strong hydrogen bonds. The ir spectra of the adduct and its deuterated counterpart have been analyzed and the stretching modes of the various hydrogen bonds identified. The1H and13C nmr. spectra are also interpreted to show that discrete adducts of U·SA persist in solution. On heating, U·SA decarboxylates at a much lower temperature than SA itself.
Similar content being viewed by others
References
Aida, K. (1963)J. Inorg. Nucl. Chem. 25, 165.
Aldrich Library of NMR Spectra (1983) 2 edn., Vol. I, p. 670B.
Äyräs, P., Laatikainen, R., and Lötjönen, S. (1980)Org. Magn. Reson. 13, 387.
Bacon, G. E., and Jude, R. J. (1973)Z. Kristallogr. 138, 19.
Barry, J. E., Finkelstein, M., Hutchins, G. A., and Ross, S. D. (1983)Tetrahedron 39, 2151.
Chang, C. (1976)J. Org. Chem. 41, 1881.
Cochran, W. (1953)Acta Cryst. 6, 260.
Colman, P. M., and Medlin, E. H. (1970)Acta Cryst. B 26, 1547.
Downie, T. C., and Speakman, J. C. (1954)J. Chem. Soc., 787.
Emsley, J. (1980)Chem. Soc. Rev. 9, 91.
Emsley, J. (1984)Struct. Bond. 57, 147.
Emsley, J., and Niazi, S. (1982)J. Chem. Soc. Dalton Trans., 2527.
Glazunov, V. P., Mashkovshii, A. A., and Odinokv, S. E. (1975)Zh. Prik. Spektrusk,23, 169.
Hadzi, D. (1965)Pure Appl Chem. 11, 435.
Hadzi, D., and Kidric, J. (1976)Spetrochim. Acta 32, 693.
Harkema, S., Bats, J. W., Weyenberg, A. W., and Feil, D. (1972)Acta Cryst. B 28, 1646.
Harkema, S., and Ter Brake, J. H. M. (1979)Acta Cryst. B 35, 1011.
Hayden, T. D., Kim, E. E., and Eriks, K. (1982)Inorg. Chem. 21, 4054.
Hsu, I. N., and Gellert, R. W. (1983)J. Crystallogr. Spectrosc. Res. 13, 43.
Kim, H. S., and Jeffrey, G. A. (1971)Acta Cryst. B 27, 1123.
Kondo, M. (1972)Bull. Chem. Soc. Jpn. 45, 2790.
Kumar, S. V., and Rao, L. M. (1982)Acta Cryst. B 38, 974.
Lu, C. S., Hughes, E. W., and Giguere, P. A. (1941)J. Am. Chem. Soc. 63, 150.
Marshall, J. L. (1983)Carbon-Carbon and Carbon-Proton NMR Couplings (Verlag Chemie International, Florida).
McCullough, J. F., Sheridan, R. C., and Frederick, L. L. (1978)J. Agric. Food Chem. 26, 670.
Molodkin, A. K., Ellert, G. V., Ivanova, O. M., and Shotnikova, G. A. (1967)Russ. J. Inorg. Chem. 12, 499.
Mootz, D., and Albrand, K. R. (1972)Acta Cryst. B 28, 2459.
Pryor, A. W., and Sanger, P. L. (1970)Acta Cryst. A 26, 543.
Radell, J., Brodman, B. W., and Domanski, J. J., Jr. (1967)J. Phys. Chem. 71, 1596.
Schaefer, T., Sebastian, R., Laatikainen, R., and Salman, S. R. (1984)Can. J. Chem. 62, 326.
Scott, K. N. (1972)J. Am. Chem. Soc. 94, 8564.
Wójcik, M. J. (1981)Chem. Phys. Lett. 83, 503.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Emsley, J., Reza, N.M. & Kuroda, R. Hydrogen bonding of urea-salicylic acid, U·SA. Journal of Crystallographic and Spectroscopic Research 16, 57–69 (1986). https://doi.org/10.1007/BF01566046
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01566046