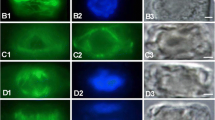
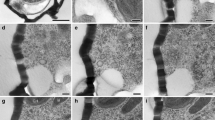
Summary
Conventional, computer-aided, and morphometric analysis of ultrathin serial sections through cells at consecutive stages of mitosis has clarified several aspects of the morphogenesis of the socalled “metaphase-band” (=MB) inChlamydomonas reinhardtii. In contrast to the original interpretation, the MB is not a single set of 4 microtubules (=MTs). This erroneous impression is created by the transient spatial association of the two parental 4-membered microtubular flagellar roots. A long section of the roots, which are constituents of the flagellar basal apparatus and the cortical cytoskeleton at interphase, persists during mitosis. Each parental 4-membered root is tightly coupled to half of the bisected basal body complex, and each daughter cell receives one root. During migration of the bisected basal body-root complexes towards the nuclear poles during prophase, the two opposite roots are bent in the middle. Starting at these bent regions, the central thirds become parallelly aligned during metaphase. The roots shorten during anaphase elongation of the nucleus, which occurs without any further change in distance between the daughter basal body-root complexes.
At anaphase de novo assembly of cytoplasmic MTs was observed. Two intracellular MT arrays, focussed at the roots like the cortical MTs, penetrate deeply into the cell. They are interpreted as the incipient cleavage MTs of the phycoplast, or radicoplast.
Similar content being viewed by others
Abbreviations
- MB:
-
metaphase band
- MT:
-
microtubule
- 3-D:
-
three-dimensional
- s:
-
sinister
References
Buffaloe ND (1958) A comparative cytological study of four species ofChlamydomonas. Bull Torrey Bot Club 85: 157–178
Burnside B (1989) Microtubule sliding and the generation of force for cell shape change. In: Warner FD, McIntosh JR (eds) Cell movement, vol 2, kinesin, dynein, and microtubule dynamics. A R Liss, New York, pp 169–189
Cavalier-Smith T (1974) Basal body and flagellar development during the vegetative cell cycle and the sexual cycle ofChlamydomonas reinhardtii. J Cell Sci 16: 529–556
Coss RA (1974) Mitosis inChlamydomonas reinhardtii basal bodies and the mitotic apparatus. J Cell Biol 63: 325–329
Deason TR, Darden WH (1971) The male initial and mitosis inVolvox. In: Parker BC, Brown RM (eds) Contributions in phycology. Allen Press, Lawrence, KS, pp 67–79
—, O'Kelley JC (1979) Mitosis and cleavage during zoosporogenesis in several coccoid green algae. J Phycol 15: 371–378
—, Ryals PE, O'Kelley JC, Bullock KW (1979) Fine structure of mitosis and cleavage inFriedmannia israelensis.(Chlorophyceae: Chlorosarcinaceae). J Phycol 15: 452–457
Doonan JH, Grief C (1987) Microtubule cycle inChlamydomonas reinhardtii. An immunofluorescence study. Cell Motil Cytoskeleton 7: 381–392
Ettl H (1975) Die GattungChlamydomonas Ehrenberg. Beihefte Nova Hedwigia 49: 1–1122
Gaffal KP (1987) Mitosis-specific oscillations of mitochondrial morphology inChlamydomonas reinhardtii. Endocytol Cell Res 4: 41–62
— (1988) The basal body-root complex ofChlamydomonas reinhardtii during mitosis. Protoplasma 143: 118–129
—, Kreutzer D (1977) The mitochondria ofPolytoma papillatum at two different stages of the vegetative cell cycle. Protoplasma 91: 167–177
Gaffal KP, Pardos D, Friedrichs GJ (1989) Effects of glucose on growth and ultrastructural phenotype ofPolytoma papillatum. Arch Protistenk 137: 99–109
—, Wolf KW, Schneider GJ (1983) Morphometric and chronobiological studies on the dynamics of the nuclear envelope and the nucleolus during mitosis of the colorless phytoflagellatePolytoma papillatum. Protoplasma 118: 19–35
Goodenough UW (1970) Chloroplast division and pyrenoid formation inChlamydomonas reinhardtii. J Phycol 6: 1–6
—, Weiss RL (1978) Interrelationships between microtubules, a striated fiber, and gametic mating structure ofChlamydomonas reinhardtii. J Cell Biol 76: 430–438
Harper JDI, John PCL (1986) Coordination of division events in theChlamydomonas cell cycle. Protoplasma 131: 118–130
Heimann K, Reize IB, Melkonian M (1989) The flagellar development cycle in algae: flagellar transformation inCyanophora paradoxa (Glaucocystophyceae). Protoplasma 148: 106–110
Holmes JA, Dutcher SK (1989) Cellular asymmetry inChlamydomonas reinhardtii. J Cell Sci 94: 273–285
Hoops HJ, Witman GB (1983) Outer doublet heterogeneity reveals structural polarity related to beat direction inChlamydomonas flagella. J Cell Biol 97: 902–908
Johnson U, Porter KR (1968) Fine structure of cell division inChlamydomonas reinhardtii: basal bodies and microtubules. J Cell Biol 38: 403–425
Lokhorst GM, Segaar PJ, Star W (1989) An ultrastructural reinvestigation of mitosis and cytokinesis in cryofixed sporangia of the coccoid green algaFriedmannia israelensis with special reference to septum formation. Cryptogam Bot 1: 275–294
Melkonian M (1980) Ultrastructural aspects of basal body associated fibrous structures in green algae: a critical review. Bio Systems 12: 85–104
— (1989) Centrin-mediated motility: a novel cell motility mechanism in eukaryotic cells. Bot Acta 102: 3–4
—, Schulze D, McFadden GI, Robenek H (1988) A polyclonal antibody (anticentrin) distinquishes between two types of fibrous flagellar roots in green algae. Protoplasma 144: 56–61
Mesquita JF, Fatima Santos M (1984) Ultrastructural study ofHaematococcus lacustris (Girod.) Rostafinski (Volvocales) II. Mitosis and cytokinesis. Cytologia (Tokyo) 49: 229–241
Moestrup O (1978) On the phylogenetic validity of the flagellar apparatus in green alga and other chlorophyll a and b containing plants. Bio Systems 10: 117–144
—, Hori T (1989) Ultrastructure of the flagellar apparatus inPyramimonas octopus (Prasinophyceae). II. Flagellar roots, connecting fibers, and numbering of individual flagella in green algae. Protoplasma 148: 41–56
Pickett-Heaps JD (1973) Cell division in Tetraspora. Ann Bot 37: 1017–1025
— (1975) Green algae. Sinauer, Sunderland, MA
Salisbury JL (1989) Algal centrin-calcium-sensitive contractile organelles. In: Coleman AW, Goff LJ, Stein Taylor JR (eds) Algae as experimental systems. Plant Biol 7: 19–37
—, Baron AT, Coling DE, Martindale VE, Sanders MA (1986) Calcium-modulated contractile proteins associated with the eukaryotic centrosome. Cell Motil Cytoskeleton 6: 193–197
—, Sanders MA, Harpst L (1987) Flagellar root contraction and nuclear movement during flagellar regeneration inChlamydomonas reinhardtii. J Cell Biol 105: 1799–1805
—, Baron AT, Sanders MA (1988) The centrin-based cytoskeleton ofChlamydomonas reinhardtii: distribution in interphase and mitotic cells. J Cell Biol 107: 635–641
Schulze D, Robenek H, McFadden GI, Melkonian M (1987) Immunolocalization of a Ca2+-modulated contractile protein in the flagellar apparatus of green algae-the nucleus-basal body connector. Eur J Cell Biol 45: 51–61
Segaar PJ, Gerritsen AF (1989) Flagellar roots as vital instruments in cellular morphogenesis during multiple fission (sporulation) in the unicellular green flagellateBrachiomonas submarina (Chlamydomonales, Chlorophyta). Cryptogam Bot 1: 279–274
— —, DeBakker MAG (1989) The cytokinetic apparatus during sporulation in the unicellular green flagellateGloeomonas kupferi-the phycoplast as a spatio-temporal differentiation of the cortical microtubule array that organizes cytokinesis. Nova Hedwigia 49: 1–23
Sluiman HJ, Blommers PCJ (1990) Basal apparatus behaviour during cellular division (sporulation) in the coccoid green algaChlorosarcina. Protoplasma 155: 66–75
Weiss RL (1984) Ultrastructure of the flagellar roots inChlamydomonas gametes. J Cell Sci 67: 133–143
Wolf KW (1984) Qualitative und quantitative Analyse ultradünner Serienschnitte durch verschiedene Entwicklungsstadien aus dem vegetativen und generativen Zellzyklus des heterotrophen PhytoflagellatenPolytoma papillatum unter besonderer Berücksichtigung des Mikrotubuliinventars. Dissertation, Universität Erlangen, Erlangen, Federal Republic of Germany
Wright RL, Salisbury J, Jarvik JW (1985) A nucleus-basal body connector inChlamydomonas reinhardtii that may function in basal body localization or segregation. J Cell Biol 101: 1903–1912
—, Adler SA, Spanier JG, Jarvik JW (1989) Nucleus-basal body connector inChlamydomonas: evidence for a role in basal body segregation and against essential roles in mitosis or determining cell polarity. Cell Motil Cytoskeleton 14: 516–526
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gaffal, K.P., el-Gammal, S. Elucidation of the enigma of the “metaphase band” ofChlamydomonas reinhardtii . Protoplasma 156, 139–148 (1990). https://doi.org/10.1007/BF01560652
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01560652