Abstract
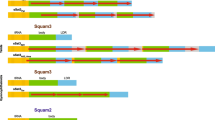
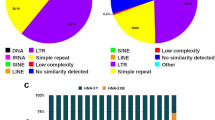
Repetitive DNA sequences were isolated from the genomes of species representing three major clades of squamate reptiles. A repetitive sequence (Cn4C7) was isolated from the New Mexican whiptail lizard,Cnemidophorus neomexicanus. This sequence is distributed throughout the chromosomes, but is more concentrated in the telomeric region. Cn4C7 also hybridizes to the chromosomes of otherCnemidophorus. Some evidence was found for concerted evolution of this repeat in hybrid unisexual lineages. In the lesser earless lizard,Holbrookia maculata, the predominant repeat in the genome is represented by a sequence (Hm1E11) which is restricted to the area flanking the centromere in all species ofHolbrookia. Two families of repetitive sequences (one dispersed, and the other telomeric) were isolated from the western diamondback rattlesnake,Crotalus atrox. The type and distribution of repetitive sequences in squamates is often taxon-specific, and may be useful as characters for elucidating taxonomic relationships.
Similar content being viewed by others
References
Arnheim N (1979) Characterization of mouse ribosomal gene fragments purified by molecular cloning.Gene 7, 83–96.
Ausubel FM, Brent R, Kingston RE,et al. (eds) (1987)Current Protocols in Molecular Biology. New York: John Wiley & Sons.
Baker RJ, Bull JJ, Mengden GA (1971) Chromosomes ofElaphe subocularis (Reptilia: Serpentes), with the description of anin vivo technique for preparation of snake chromosomes.Experientia 27, 1228–1229.
Baker RJ, Wichman HA (1990) RetrotransposanMys is concentrated on the sex chromosomes: Implications for copy number containment.Evolution 44, 2083–2088.
Bogenberger JM, Neitzel H, Fittler F (1987) A highly repetitive DNA component common to all Cervidae: its organization and chromosomal distribution during evolution.Chromosoma 95, 154–161.
Bradley RD, Davis SK, Bayouth JM, Hamilton MJ, Maltbie M, Baker RJ (1991) Chromosomal distribution of some repetitive DNA sequences in pocket gophers (Geomys, Crato-geomys, and Thomomys) as determined byin situ hybridi-zation.Occas Pap, Mus, Texas Tech Univ 141, 1–14.
Capriglione T, Olmo E, Odierna G, Smith DI, Miller OJ (1989) Genome composition and tandemly repetitive sequence at some centromeres in the lizardPodarcis s. sicula Raf.Genetica 79, 85–91.
Demas S, Wachtel S (1991) DNA fingerprinting in reptiles: Bkm hybridization patterns in Crocodilia and Chelonia.Genome 34, 472–476.
de Queiroz K (1992) Phylogenetic relationships and rates of allozyme evolution among lineages of sceloporine sand lizards.Biol. J. Linn. Soc. 45, 333–362.
Epplen JT, Diedrich UD, Wagenmann M, Schmidtke J, Engel W (1979) Contrasting DNA sequence organization patterns in sauropsidian genomes.Chromosoma 75, 199–214.
Etheridge R, de Queiroz K (1988) A phylogeny of Iguanidae. In: Estes R and Pregill G, eds.Phylogenetic Relationships of the Lizard Families. Essays Commemorating Charles L. Camp. Stanford, California: Stanford University Press, pp 283–367.
Evans GA, Lewis K, Rothenberg BE (1989) High efficiency vectors for cosmid microcloning and genomic analysis.Gene 79, 9–20.
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity.Anal Biochem 132, 6–13.
Frost DR, Etheridge R (1989) A phylogenetic analysis and taxonomy of iguanian lizards (Reptilia: Squamata).Misc Publ Univ Kansas Mus Nat Hist 81, 1–65.
Hamilton MJ, Honeycutt RL, Baker RJ (1990) Intragenomic movement, sequence amplification and concerted evolution in satellite DNA inReithrodontomys: Evidence fromin situ hybridization.Chromosoma 99, 321–329.
Hamilton MJ, Hong G, Wichman HA (1992) Intragenomic movement and concerted evolution of satellite DNA inPeromyscus: evidence fromin situ hybridization.Cytogenet Cell Genet 60, 40–44.
Hillis DM, Moritz C, Porter CA, Baker RJ (1991) Evidence for biased gene conversion in concerted evolution of ribosomal DNA.Science 251, 308–310.
Janecek LL, Longmire JL, Wichman HA, Baker RJ (1993) Genome organization of repetitive elements in the rodent,Peromyscus leucopus.Mammalian Genome 4, 374–381.
Jones KW, Singh L (1981) Conserved repeated DNA sequences in vertebrate sex chromosomes.Hum Genet 58, 46–53.
Knight A, Densmore LD III, Rael ED (1992) Molecular systematics of the Agkistrodon complex. In: Campbell JA and Brodie ED, Jr, eds.Biology of the pitvipers. Tyler, Texas: Selva, pp 49–69.
Leviton AE, Gibbs RH, Jr, Heal E, Dawson CE (1985) Standards in herpetology and ichthyology: Part I. Standard symbolic codes for institutional resource collections in herpetology and ichthyology.Copeia 1985, 802–832.
Longmire JL, Lewis AK, Brown NC,et al. (1988) Isolation and molecular characterization of a highly polymorphic centromeric tandem repeat in the family Falconidae.Genomics 2, 14–24.
Love J, Deininger P (1992) Characterization and phylogenetic significance of a repetitive DNA sequence from whooping cranes (Grus americana).Auk 109, 73–79.
Meyne J, Baker RJ, Hobart HH,et al. (1990) Distribution of nontelomeric sites of the (TTAGGG) n telomeric sequence in vertebrate chromosomes.Chromosoma 99, 3–10.
Minnick MF, Stillwell LC, Heineman JM, Stiegler GL (1992) A highly repetitive DNA sequence possibly unique to canids.Gene 110, 235–238.
Monaco PJ, Swan KF, Rasch EM, Musich PR (1989) Characterization of a repetitive DNA in the unisexual fishPoecilia formosa. I. Isolation and cloning of the MboI family,In: Dawley RM and Bogart JP, eds.Evolution and Ecology of Unisexual Vertebrates. Bull. 466, Albany, New York State Museum, pp 123–131.
Moyzis RK, Albright KL, Bartholdi MF,et al. (1987) Human chromosome-specific repetitive DNA sequences: Novel markers for genetic analysis.Chromosoma 95, 375–386.
Naruse K, Mitani H, Shima A (1992) A highly repetitive interspersed sequence isolated from genomic DNA of the Medaka,Oryzias latipes, is conserved in three other related species within the genusOryzias.J Exp Zool 262, 81–86.
Olmo E (1981) Evolution of genome size and DNA base composition in reptiles.Genetica 57, 39–50.
Olmo E, Capriglione T, Odierna G (1989) Genome size evolution in vertebrates: Trends and constraints.Comp Biochem Physiol 92B, 447–453.
Paull D, Williams EE, Hall WP (1976) Lizard karyotypes from the Galapagos islands: chromosomes in phylogeny and evolution.Breviora 441, 1–31.
Peacock WJ, Dennis ES, Elizur A, Calaby JH (1981) Repeated DNA sequences and kangaroo phylogeny.Aust J Biol Sci 34, 325–340.
Porter CA, Hamilton MJ, Sites JW, Jr, Baker RJ (1991) Location of ribosomal DNA in chromosomes of squamate reptiles: systematic and evolutionary implications.Herpetologica 47, 271–280.
Porter CA, Sites JW, Jr (1986) Evolution ofSceloporus grammicus complex (Sauria: Iguanidae) in central Mexico: Population cytogenetics.Syst Zool 35, 334–358.
Porter CA, Haiduk MW, de Queiroz K (1994) Evolution and phylogenetic significance of ribosomal gene location in chromosomes of squamate reptiles.Copeia in press.
Quinn JS, Guglich E, Seutin Get al. (1992) Characterization and assessment of an avian repetitive DNA sequence as an icterid phylogenetic marker.Genome 35, 155–162.
Sambrook J, Fritsch EF, Maniatis T (1989)Molecular Cloning: A Laboratory Manual. Second edition. Cold Spring Harbor Laboratory Press, Plainview, New York.
Schmidtke J, Epplen JT (1980) Sequence organization of animal nuclear DNA.Hum Genet 55, 1–18.
Singer M, Berg P (1991)Genes and Genomes. Mill Valley, California: University Science Books.
Sites JW, Jr, Bickham JW, Haiduk MW, Iverson JB (1979) Banded karyotypes of six taxa of kinosternid turtles.Copeia 1979, 692–698.
Soma M, Beçak ML, Beçak W (1975) Estudo comparativo do conteúdo de DNA em 12 espécies de lacertílios.Ciência e Cultura 27, 1324–1327.
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis.J Mol Biol 94, 503–517.
Van Den Bussche RA (1991) Phylogenetic analysis of restriction site variation in the ribosomal DNA complex of New World leaf-nosed bat genera.Syst Zool 40, 420–432.
Van Den Bussche RA (1992) Restriction-site variation and molecular systematics of New World leaf-nosed bats.J Mammal 73, 29–42.
Van Den Bussche RA, Baker RJ, Wichman HA, Hamilton MJ (1993) Molecular phylogenetics of stenodermatini bat genera: Congruence of data from nuclear and mitochondrial DNA.Mol Biol Evol 10, 944–959.
Wiens, JJ (1993) Phylogenetic relationships of phrynosomatid lizards and monophyly of theSceloporus group.Copeia 1993, 287–299.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Porter, C.A. Organization and chromosomal location of repetitive DNA sequences in three species of squamate reptiles. Chromosome Res 2, 263–273 (1994). https://doi.org/10.1007/BF01552720
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01552720