Abstract
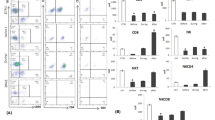
In the present study we tested the phenotypic profile as well as several immunological responses of peripheral blood mononuclear cells (PBMC) isolated from melanoma patients. These patients underwent chemotherapy with dacarbazine and carboplatin from day 1 to day 22, followed by immunotherapy of low-dose recombinant interleukin-2 and recombinant interferon α administered subeutaneously from day 36 to day 75. The PBMC from 14 patients were isolated on day 0 before chemotherapy. on day 36 after chemotherapy and on day 76 after immunotherapy. After chemotherapy, a decrease in CD16+ cells and increase in CD3+ and CD4+ cells correlated with a significant decrease in the generation of lymphokine-activated killer (LAK) activity. After immunotherapy, an increase in CD16+ cells correlated with an increase in the induction of LAK activity. A comparison between responding and non-responding patients revealed statistically significant differences in LAK activity of PBMC and response to concanavalin A following chemotherapy, and in the percentage of CD8+ cells following immunotherapy. Our results point toward the value of continuing such a study on a larger population of cancer patients in order to select the appropriate bioassays for monitoring and predicting the clinical responsiveness to combined therapies.
Similar content being viewed by others
References
Atzpodien J, Korfer A, Evers P, Franks CR, Knuver-Hopf J, Lopez-Hanninen E, Fischer M, Mohr H, Dallmann I, Hadam M, Hubert P, Kirchner H (1990) Low-dose subcutaneous recombinant interleukin-2 in advanced human malignancy: a phase II outpatient study. Mol Biother 2: 18
Ballas ZK, Rasmussen W (1987) Lymphokine-activated killer (LAK) cells. III. Characterization of LAK precursors and susceptible target cells within the murine thymus. J Immunol 139: 3542
Bloomgren H, Anderson B (1969) Evidence for a small pool of immunocompetent cells in the mouse thymus. Exp Cell Res 57: 185
Brunda MJ, Bellantoni D, Sulich V (1987) In-vivo anti-tumor activity of combinations of interferon-alpha and interleukin-2 in a murine model. Correlation of efficacy with the induction of cytotoxic cells resembling natural killer cells. Int J Cancer 40: 365
Cameron RB, McIntosh JK, Rosenberg SA (1988) Syngeneic antitumor effects of combination immunotherapy with recombinant interleukin-2 and recombinant hybrid alpha-interferon in the treatment of established murine hepatic metastases. Cancer Res 48: 5810
Eisenthal A, Rosenberg SA (1989) The effect of various cytokines on the in vitro induction of ADCC in murine cells: enhancement of the IL-2 induced ADCC activity by IL-1. J Immunol 142: 2307
Eisenthal A, Cameron RB, Rosenberg SA (1990) Induction of ADCC in vivo by alpha-IFN and its anti-tumor efficacy against established B16 melanoma liver metastases when combined with specific anti-B16 monoclonal antibody. J Immunol 144: 4463
Fidler JJ, Heicappell R, Saiki I, Grutter G, Horisberger MA, Nuesch J (1987) Direct antiproliferative effects of recombinant human interferon-alpha B/D hybrids on human tumor cell lines. Cancer Res 47: 2020
Gehan EA (1965) Generalized Wilcoxon test for comparing arbitrarily single censored samples. Biometrica 52: 203
Grimm EA, Mazunder A, Zhang HZ, Rosenberg SA (1982) The lymphokine activated killer cell phenomenon: lysis of NK resistant fresh solid tumor cells by IL-2 activated autologous human peripheral blood lymphocytes. J Exp Med 155: 1823
Herman J, Dinarello CA, Kew MC, Rabson AR (1985) The role of interleukin-1 (IL-1) in tumor-NK cell interactions: correlation of defective NK cell activity in cancer patients by treating target cells with IL-1. J Immunol 135: 2882
Huberman M, Bering H, Fallon B, Tessitore J, Sonnenborn H, Paul S, Zeffren J, Levitt D, Groopman J (1991) A phase I study of recombinant human interleukin-2 and alpha 2 a-intefeeron in patients with solid tumors. Cancer 68: 1708
Itoh K, Tilden AR, Kumagai K, Balch CM (1985) Leull-lymphocytes with natural killer (NK) activity are precursors of recombinant IL-2 (rII2)-induced activated killer (AK) cells. J Immunol 134: 802
Kiyohara T, Taniguchi K, Kubota S, Koga S, Sakuragi T, Saitoh Y (1988) Inducaion of lymphokine-activated killer-like cells by cancer chemotherapy. J Exp Med 168: 2355
Lanier LL, Benike CJ, Phillips JH, Engelman EG (1985) Recombinant interleukin 2 enhanced natural killer cell-mediated cytotoxicity in human lymphocyte subpopulations expressing the Leu 7 and Leu 11 antigens. J Immunol 134: 794
Lotze MT, Grimm EA, Mazumder A, Rosenberg SA (1981) In vitro growth of cytotoxic human T-lymphocytes. IV. Lysis of fresh and cultured autologous tumor by lymphocytes cultured in T-cell growth factor (TCGF). Cancer Res 41: 4420
Matsumoto S, Moriyama M, Imanishi H (1987) Effects of recombinant human interferon-gamma (Met-Gln form) on expression of Fc receptor and Ia-like antigen of human peripheral monocytes and lymphocytes: a comparative study with natural human interferon-alpha and-beta. Chem Pharm Bull (Tokyo) 35: 355
Mule JJ, Shu S, Schwartz SL, Rosenberg SA, (1984) A successful adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant IL-2. Science 225: 1487
O'Flynn K, Krensky AM, Beverley PCL, Burakoff SJ, Linch DC (1985) Phytohaemaglutinin activation of T cells through the sheep red blood receptor. Nature 313: 686
Papie'ernik M, Laroche L, Bach JF (1977) Thymocyte subpopulations in young and adult mice. I Separation by density gradient and steroid treatment. Eur J Immunol 7: 796
Paucker K, Cantell K, Henle W (1962) Quantitative studies on viral interference in suspended L cells. III. Effect of interfering viruses and interferon on the growth rate of cells. Virology 17: 324
Phillips JH, Lanier LL (1986) Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med 164: 814
Roberts K, Lotze MT, Rosenberg SA (1986) Separation and functional studies of the human lymphokine-activated killer cell. Cancer Res 47: 4366
Rosenberg SA, Spiess PJ, Lafreniere R (1986) A new approach to the adoptive immunotherapy of cancer with tumor infiltrating lymphocytes. Science 233: 1318
Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Seipp CA, Simpson CG, White DE (1987) A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 316: 889
Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, White DE (1989) Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg 210: 474
Talmadge JE, Herberman RB, Chirigos MA, Maluish AE, Schneider MA, Adams JS, Phillips H, Thurman GB, Varesio L, Long CW, Oldham RK, Wiltrout RH (1985) Hyporesponsiveness to augmentation by multiple injections of various immunomodulators including recombinant interferons and interleukin-2. J Immunol 135: 2483
Topalian SL, Solomon D, Avis FP, Chang AE, Freerksen DL, Linehan WM, Lotze MT, Robertson CN, Seipp CA, Simon P, Simpson CG, Rosenberg SA (1988) Immunotherapy of patients with advanced cancer using tumor infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol 6: 839
Topalian SL, Solomon D, Rosenberg SA (1989) Tumor specific cytolysis by lymphocytes infiltrating human melanoma. J Immunol 142: 3714
Trunch A, Albert F, Golstein P, Schmitt-Verhulst AM (1983) Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature 313: 318
Van den Berg HW, Leahey WJ, Lynch M, Clarke R, Nelson J (1987) Recombinant human interferon alpha increases oestrogen receptor expression in human breast cancer cells (ZR-75-1) and sensitizes them to the anti-proliferative effects of tamoxifen. Br J Cancer 55: 255
Wan YJ, Orisson BM, Lieberman R, Lazarovici P, Ozato K (1987) Induction of major histocompatibility class I antigens by interferons in undifferentiated F9 cells. J Cell Physiol 130: 276
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eisenthal, A., Skornick, Y., Ron, H. et al. Phenotypic and functional profile of peripheral blood mononuclear cells isolated from melanoma patients undergoing combined immunotherapy and chemotherapy. Cancer Immunol Immunother 37, 367–372 (1993). https://doi.org/10.1007/BF01526792
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01526792