Abstract
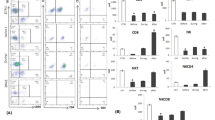
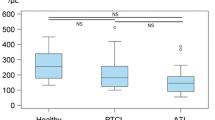
We previously found that the ability of peripheral blood mononuclear cells (PBM) of cancer patients to generate lymphokine-activated killer (LAK) cells became remarkably augmented after mitomycin C administration. On the basis of the clinical finding, we designed a treatment regimen comprised of 12 mg/m2 mitomycin C i. v. on day 1 and 700 U/m2 recombinant interleukin-2 (IL-2) i.v. every 12 h from day 4 through day 8. Of 25 patients with advanced carcinoma, 9 had a partial response and 3 had a minor response. Cytotoxic cell function, including natural killer activity, lymphokine-activated killer (LAK) activity, and the ability to generate LAK cells, and lymphocyte subsets in PBM was measured 1 day before and after either the first or second course of this therapy. The relationship between these parameters and the clinical antitumor response to this treatment was examined. Although the cytotoxic activities were significantly augmented after either the first or second treatment course, no positive correlation was observed between the changes in these cytotoxic activities and the clinical response to this therapy, when patients who either showed a partial response or whose disease remission was partial or minor were defined as responders. Further, phenotypic analysis showed a significant increase in CD2+, CD3+ CD4+ and CD4+Leu8− cells after the firs course, and CD25+ cells after either the first or second course of this treatment. The precentages of CD2+ and CD25+ cells were significantly elevated only in responders but not in nonresponders, suggesting the increase in these subsets was related to clinical response.
Similar content being viewed by others
References
Akiyoshi T, Arinaga S, Karimine N, Inoue H, Abe R, Takamuku K, Watanabe D, Nagamatsu M, Matsuoka H, Ueo H (1990) Effect of recombinant interleukin 2 in combination with mitomycin C or adriamycin on advanced cancer. Proc. Am Assoc Cancer Res 31: 273
Akiyoshi T, Arinaga S, Nanbara S, Karimine N, Inoue H, Takamuku K, Abe R, Watanabe D, Nagamatsu M, Matsuoka H, Ueo H (1990) The effect of interleukin 2 in combination with mitomycin C on advanced cancer. Jpn. J Surg 20: 365
Arinaga S, Karimine, N, Takamuku K, Nanbara S, Inoue H, Abe R, Watanabe D, Matsuoka H, Ueo H, Akiyoshi T (1992) Correlation of eosinophila with clinical response in patients with advanced carcinoma treated with low-dose recombinant interleukin-2 and mitomycin C. Cancer Immunol Immunother 35: 246
Blay J-Y, Favrot MC, Negrier S, Cambaret V, Chouaib S, Mercatellow A, Maemmerlen P, Franks CR, Philip T (1990) Correlation between clinical response to interleukin 2 therapy and sustained production of tumor necrosis factor. Cancer Res 50: 2371
Caligiuri MA, Murray C, Soiffer RJ, Klumpp TR, Seiden M, Cochran K, Cameron C, Ish C, Buchanan L, Perillo D, Smith K Ritz J (1991). Extended continuous infusion low-dose recombinant interleukin-2 in advanced cancer: prolonged immunomodulation without significant toxicity. J Clin Oncol 9: 2110
Cohen PJ, Lotze MT, Roberts JR, Rosenberg SA, Jaffe ES (1987) The immunopathology of sequential tumor biopsies in patients treated with interleukin-2. Correlaton of response with T-cell infiltration and HLA-DR expression. Am J Pathol 129: 208
Creekmore SP, Harris JE, Ellis TM, Braun DP, Cohen II, Bhoopatam NB, Jassak PF, Cahill MA, Canzoneri CL, Fisher RI (1989) A phase I clinical trial of recombinant interleukin 2 by periodic 24-hour intravenous infusions. J Clin Oncol 7: 276
Eberlein TJ, Rodrick ML, Massaro AF, Jung S-E, Mannick JA, Schoof DD (1989) Imunomodulatory effects of systemic low-dose recombinant interleukin-2 and lymphokine-activated killer cells in humans. Cancer Immunol Immunother 30:145
Favrot MC, Combaret V, Negrier S, Philip I, Thiesse P, Freydel C, Bijmann JT, Franks CR, Mercatello A, Philip T (1990) Functional and immunophenotypic modifications induced by interleukin-2 did not predict response to therapy in patients with renal cell carcinoma. J Biol Response Mod 9: 167
Gambacorti-Passerini C, Radrizzani M, Marolda R, Belli G (1988) In vivo activation of lymphocytes in melanoma patients receiving esclating doses of recombinant interleukin 2. Int J Cancer 41: 700
Gemlo BT, Palladino MA, Jaffe HS, Espevik TP, Rayner AA (1988) Circulating cytokines in patients with metastatic cancer treated with recombinant interleukin 2 and lymphokine-activated killer cells. Cancer Res 48: 5864
Ghosh AK, Dazzi H, Thatcher N, Moore M (1989) Lack of correlation between peripheral blood lymphokine-activated killer (LAK) cell function and clinical response in patients with advanced malignant melanoma receiving recombinant interleukin 2. Int J Cancer 43: 410
Goldstein D, Sosman JA, Hank JA, Weil-Hillman G, Moore KH, Borchert A, Bechhofer R, Storer B, Kohler PC, Levitt D, Sondel PM (1989) Repetitive weekly cycles of interleukin 2: effect of outpatient treatment with a lower dose of interleukin 2 on non-major histocompatibility complex-restricted killer activity. Cancer Res 49: 6832
Hanninen EL, Korfer A, Hadam M, Schneekloth C, Dallmann I, Manzel T, Kirchner H, Poliwoda H, Atzpodien J (1991) Biological monitoring of low-dose interleukin 2 in humans: soluble interleukin 2 receptors, cytokines, and cell surface phenotypes. Cancer Res 50: 6312
Hermann GG, Geertsen PF, Masse H, Zeuthen J (1991) Interleukin-2 dose, blood monocyte and CD25+ lymphocyte counts as predictors of clinical response to interleukin 2 therapy in patients with renal cell carcinoma. Cancer Immunol Immunother 34: 111
Hermann GG, Geertsen PF, Maase H, Steven K, Andersen C, Hald T, Zeuthen J (1992) Recombinant interleukin-2 and lymphokine-activated killer cell treatment of advanced bladder cancer: clinical results and immunological effects. Cancer Res 52: 726
Hinuma S, Onda H, Naruo K, Ichimori Y, Koyama M, Tsukamoto K (1982) Translation of interleukin 2 mRNA from human peripheral blood leukocytes inXenopus oocytes. Biochem Biophys Res Commun 109: 363
Kasid A, Director EP, Rosenberg SA (1989) Induction of endogenous cytokine-mRNA in circulating peripheral blood mononuclear cells by IL-2 administration to cancer patients. J Immunol 143: 736
Krigel RL, Padavic-Shaller KA, Rudolph AR, Konrad M, Bradley EC, Comis RL (1990) Renal cell carcinoma: treatment with recombinant interleukin-2 plus beta interferon. J Clin Oncol 8: 460
Lotze MT, Custer MC, Sharrow SO, Rubin LA, Nelson DL, Rosenberg SA (1987) In vivo administration of purified human interleukin-2 to patients with cancer: development of interleukin-2 receptor positive cells and circulating soluble interleukin-2 receptors following interleukin-2 administration. Cancer Res 47: 2188
Mitchell MS, Kempf RA, Harel W, Shau H, Boswell WD, Lind S, Bradley EC (1988) Effectiveness and tolerability of low-dose cyclophosphamide and low-dose intravenous interleukin-2 disseminated melanoma. J Clin Oncol 6: 409
Mule JJ, Yang JC, Lafreniere S, Shu S, Rosenberg SA (1987) Identification of cellular mechanisms operational in vivo during the regression of established pulmonary metastases by systemic administration of high-dose recombinant interleukin-2. J Immunol 139: 285
Nanbara S, Arinaga S, Akiyshi T (1989) Augmentation of the generation of lymphokine-activated killer cells after a single dose of mitomycin C in cancer patients. Cancer Immunol Immunother 29: 237
Park KGH, Heys DS, Murray JB, Hays PD, Ashoby JA, Franks CR (1992) Recombinant interleukin-2 treatment in patients with metastatic colorectal cancer: effect on natural cytotoxicity. Cancer Immunol Immunother 35: 53
Peace DJ, Cheever MA (1989) Toxicity and therapeutic efficacy of high-dose interleukin-2. J Exp Med 169: 161
Phillips JH, Gemlo BT, Myers WW, Rayner AA, Lanier LL (1987) In vivo and in vitro activation of natural killer cells in advanced cancer patients undergoing combined interleukin-2 and LAK cell therapy. J Clin Oncol 5: 1933
Rosenberg SA, Lotze MT, Muul JM, Chang AE, Avis EP, Leitman S, Linchan WM, Robertson CN, Lee RE, Rubin JT, Seipp CA, Simpson CG, White MS (1987) A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high dose IL 2 alone. N Engl J Med 316: 889
Rubin JT, Elwood LJ, Rosenberg SA, Lotze MT (1989) Immunohistochemical correlates of response to recombinant interleukin-2-based immunotherapy in humans. Cancer Res 49: 7086
Taguchi T, Kimoto Y (1987) Clinical application of biological response modifiers: interleukin 2 (in Japanese) Saishin-Igaku. 42: 325
Thompson JA, Lee DJ, Lindgren CG, Benz LA, Collins C, Levitt D, Fefer A (1988) Influence of dose and duration of infusion of interleukin-2 on toxicity and immunomodulation. J Clin Oncol 6: 669
Urba WJ, Steis RG, Longo DL, Kopp WC, Maluish AE, Marcon L, Nelson DL, Stevenson HC, Clarks JW (1990) Immunomodulatory properties and toxicity of interleukin 2 in patients with cancer. Cancer Res 50: 185
Weidmann E, Bergmann L, Hechler P, Mitrou PS (1991) Cytotoxic activity and phenotypic characteristics of lymphocyte subsets after therapy of cancer patients with interleukin-2.Cancer Immunol Immunother 33:398
West WH, Tanner KW, Yanelli JR, Marshall GD, Orr DW, Thurmann GB, Oldham RT (1987) Constant infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med 316: 398
Yamaguchi Y, Suda T, Shiozaki H, Miura Y, Hitoshi Y, Tominaga A, Takatsu K, Kasahara T (1990) Role of IL-5 in IL-2-induced eosinophilia: in vivo and in vitro expression of IL-5 mRNA by IL-2. J Immunol 145: 873
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arinaga, S., Karimine, N., Adachi, M. et al. Cytotoxic cell function and phenotypic analysis of peripheral blood mononuclear cells in cancer patients treated with low-dose interleukin-2 and mitomycin C. Cancer Immunol Immunother 37, 220–226 (1993). https://doi.org/10.1007/BF01518514
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01518514