Summary
Adsorption isotherms of water, n-butane, i-butane, benzene and cumene on synthetic and natural mordenites were determined by the gravimetric method and the effect of exchangeable cations on the adsorption character of mordenite was discussed.
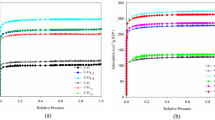
In the case of synthetic “Large-Port Mordenite”, even its Na-form adsorbs benzene or cumene, whereas the natural mordenite is a “Small-Port Mordenite” and its Na-form adsorbs n-paraffin but it does not adsorb benzene. NH4-form of the natural mordenite, whose NH4-exehange ratio is sufficiently high, adsorbs cumene, but the amount of cumene adsorbed is smaller than that of the synthetic Na-form. This result indicates that the pore diameter of the natural NH4-form is smaller than that of “Large-Port Mordenite”.
It may be considered that two causes will disturb the adsorption of large molecules on “Small-Port Mordenite”; one is the reduction of main channel radius by exchanged cations and the other the existence of amorphous substance.
Zusammenfassung
Die Adsorptionsisothermen von Wasser, n-Butan, Isobutan, Benzol und Cumol an den Natur- und synthetischen Mordeniten wurden mit der gravimetrischen Methode gemessen. Die Beziehung der Menge der austauschbaren Kationen mit der Adsorptionsfähigkeit der Mordenite wird diskutiert. Beim synthetischen Mordenit ist die Na-Form schon fähig, Benzol oder Cumol zu adsorbieren. Der Naturmordenit gehört dagegen dem „small-port“-Typ an. Die Na-Form vom Naturmordenit, sofern die Menge von ausgetauschtem NH4 hinreichend groß ist, adsorbiert Cumol. Aber die Menge des adsorbierten Cumols ist geringer als bei der synthetischen Na-Form.
Diese Tatsache weist darauf hin, daß bei der NH4-Form vom Naturmordenit der Durchmesser der Poren kleiner ist als bei dem „large-port“-Typ.
Um zu erklären, was die Adsorption von großen Molekülen hindert, werden zwei mögliche Deutungen angegeben, nämlich der von ausgetauschten Kationen verkleinerte Querschnitt von Kanälen und das Vorhandensein amorpher Substanzen.
Similar content being viewed by others
References
Meier, W. M., Z. Krist.115, 439 (1961).
Barrer, R. M., Trans. Faraday Soc.45, 358 (1949).
Barrer, R. M., Brit. Chem. Eng.4, 267 (1959).
Barrer, R. M., J. Chem. Soc.1948, 2158.
Keough, A. H. andL. B. Sand, J. Amer. Chem. Soc.83, 3536 (1961).
Barrer, R. M. andD. L. Peterson, Proc. Roy. Soc. A,280, 466 (1969).
Frilette, V. J. andM. K. Rubin, J. Cat.4, 310 (1965).
Sudo, T., H. Hayashi, T. Nishiyama, andK. Chin, Nendo-Kagaku (J. Clay Sci. Soc. Japan)2, 126 (1963).
Domine, D. andJ. Quobex, Preprint of Molecular Sieve Symposium held at London, p-70 (1967).
Eberly, Jr.,P. E., J. Phys. Chem.67, 2404 (1963).
Sand, L. B., Preprint of Molecular Sieve Symposium held at London, p-33 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nishimura, Y., Takahashi, H. Effects of exchangeable cations on the adsorption character of mordenite. Kolloid-Z.u.Z.Polymere 245, 415–419 (1971). https://doi.org/10.1007/BF01501006
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01501006