Summary
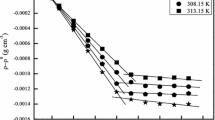
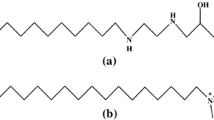
The critical micelle concentrations of octylbetaine and N-octylbetaine were determined by three independent techniques; surface tension, density and light-scattering measurements. The values obtained were 0.097, 0.125, 0.120 mole/l for octylbetaine and 0.250, 0.250, 0.217 mole/l for N-octylbetaine. The micellar weights of these surfactants were estimated from the light-scattering data. These were 7.04×103 for the former and 5.10×103 for the latter.
Zusammenfassung
Die kritischen Mycellkonzentrationen von Octylbetain und N-Octylbetain wurden auf drei unabhängige Weisen bestimmt. durch Messung der Oberflächenspannung, Dichte und Lichtstreuung. Die erhaltenen Werte sind 0,097, 0,125, 0,120 Mol/l für Octylbetain und 0,250, 0,250, 0,217 Mol/l für N-Octylbetain. Die Mycellargewichte dieser grenzflächenaktiven Substanzen lassen sich aus der Lichtstreuung abschätzen. Sie betragen 7,04 · 103 für die erstere und 5,10 · 103 für die letztere Substanz.
Similar content being viewed by others
References
Tori, K. andT. Nakagawa, (Kolloid-Z. u. Z. Polymere187, 44 (1963).
Kusano, T. andJ. Mikumo, Kôgyô Kagaku Zasshi59, 458 (1956).
Shinoda, K. andK. Mashio, J. Phys. Chem.64, 54 (1960).
Harkins, W. D. andF. E. Brown, J. Amer. Chem. Soc.41, 499 (1919).
Shinoda, K., T. Yamaguchi andR. Hori, Bull. Chem. Soc. Japan34, 237 (1961).
Bury, C. R. andJ. Browning, Trans. Faraday Soc.49, 209 (1953).
Nilsson, G., J. Phys. Chem.61, 1135 (1957).
Wilson, A., M. B. Epstein andJ. Ross, J. Colloid Sci.12, 345 (1957).
McBain, M. E. L. andE. Hutchinson, Solubilization and Related Phenomena, p. 32 (New York 1955).
Debye, P., J. Phys. Colloid Chem.53, 1 (1949), Ann. N. Y. Acad. Sci.51, 575 (1949).
Shinoda, K., T. Yamanaka andK. Kinoshita, J. Phys. Chem.63, 648 (1959).
Clarke, H. T., J. Chem. Soc.103, 1689 (1913).
Tanaka, F. andY. Kato, Yakugaku Zasshi (J. Pharm. Soc. Japan)63, 592 (1943).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tori, K., Nakagawa, T. Colloid chemical properties of ampholytic surfactants. Kolloid-Z.u.Z.Polymere 188, 47–52 (1963). https://doi.org/10.1007/BF01499603
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01499603