Summary
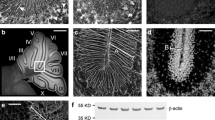
A rat monoclonal antibody (OZ42), raised against immature mouse granule cells, recognizes a region of the external granular layer of postnatally developing cerebellar cortex. This region, about three cells thick, is adjacent to the developing molecular layer and contains postmitotic, premigratory granule cells. The OZ42 reactivity commenced near postnatal day 3 (P3), the deep external granular layer was strongly reactive by P10 and this level was maintained while granule cells remained in the external granular layer (approximately P15). Isolated immature granule cells in cytospin preparations specifically reacted with OZ42. Reactivity was extranuclear and was substantially reduced when cells were prepared by trypsinization, suggesting that at least some of the antigen is associated with the outer surface of the plasma membrane. Other postnatal reactivity to OZ42 (P0 to P3) was found in a band of cells in the deep cortical layers overlying the corpus callosum through the entorhinal cortex, terminating adjacent to the hippocampus. Reactivity in some regions of the corpus callosum and anterior commissure was seen from P0 to P5. No reactivity of non-neural tissues was observed at any stage. In the embryo there was extensive staining of the CNS and PNS at E10 and E14, which was largely gone by E16. Weaver mutant mice examined for reactivity to OZ42 showed that the granule cell death and cerebellar disorganization in P10 homozygous mutants was associated with a substantial decrease in OZ42 reactivity in the external granular layer. At P14 and P20, OZ42 reactivity in the weaver external granular layer was restricted to single cells, rather than an entire layer of cells, further indicating that the OZ42 antigen is present on granule cells rather than the substratum. By Western analysis of non-reducing SDS-PAGE gels, OZ42 recognized a single band with the molecular weight between 120 and 145 kD in P10, but not adult cerebellum of BALB/c mice. An OZ42-specific band at 60–70 kD was also seen under reducing conditions and occasionally in non-reducing conditions. These bands were not recognized by antibodies against NCAM, L1 and AMOG. Immunoprecipitation and cross-blocking with antiserum to TAG-1 suggested that OZ42 recognized the same molecule in the mouse cerebellum that has been described in embryonic rat and mouse spinal cord. The developmentally regulated expression of the neural-specific molecule recognized by OZ42 in the postnatal cerebellum suggests it may be involved with the early stages of granule cell axon elongation.
Similar content being viewed by others
References
ALTMAN, J. (1972) Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and transitional molecular layer.Journal of Comparative Neurology 145, 353–98.
ANTONICEK, H., PERSOHN, E. & SCHACHNER, M. (1987) Biochemical and functional characterization of a novel neuron—glia adhesion molecule that is involved in neuronal migration.Journal of Cell Biology 104, 1587–95.
BASTIANI, M. J., HARRELSON, A. I., SNOW, P. M. & GOODMAN, C. S. (1987) Expression of Fasciclin I and II glycoproteins on a subset of neurons and axon pathways inDrosophila.Cell 48, 975–88.
BOLIN, L. M. & ROUSE, R. V. (1986) Localization of Thy-1 expression during postnatal development of the mouse cerebellar cortex.Journal of Neurocytology 15, 29–36.
BOLIN, L. M., THOMAS, A. R., MAYER, D. N. & ROUSE, R. V. (1988) Independent developmental regulation of migratory granule neurons and their cerebellar ligand in the mouse.Neuroscience Letters 85, 158–62.
BURGOYNE, R. D. & CAMBRAY-DEAKIN, M. A. (1988) The cellular neurobiology of neuronal development: the cerebellar granule cell.Brain Research Reviews 13, 77–101.
CALVERT, R. & ANDERTON, B. H. (1985) A microtubule-associated protein (MAP1) which is expressed at elevated levels during development of the rat cerebellum.EMBO Journal 4, 1171–6.
CHUN, J. J. M. & SHATZ, C. J. (1988) A fibronectin-like molecule is present in the developing cat cerebral cortex and is correlated with subplate neurons.Journal of Cell Biology 106, 857–72.
CHUONG, C.-M., CROSSIN, K. L. & EDELMAN, G. M. (1987) Sequential expression and differential function of multiple adhesion molecules during formation of cerebellar cortical layers.Journal of Cell Biology 104, 331–42.
DODD, J., MORTON, S. B., KARAGOGEOS, D., YAMAMOTO, M. & JESSELL, T. M. (1988) Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons.Neuron 2, 105–16.
EDELMAN, G. (1986) Cell adhesion in neural histogenesis.Annual Reviews in Physiology 48, 417–30.
EDMONSON, J. C., LIEM, R. K. H., KUSTER, J. E. & HATTEN, M. E. (1988) Astrotactin: a novel neuronal cell surface antigen that mediates neuron—astroglial interactions in cerebellar microcultures.Journal of Cell Biology 106, 505–17.
FAISSNER, A., KRUSE, J., NIEEKE, J. & SCHACHNER, M. (1984) Expression of neural adhesion molecule L1 during development, in neurological mutants and in the peripheral nervous system.Developmental Brain Research 15, 69–82.
GHANDOUR, M. S., FOUCAUD, B. & GOMBOS, G. (1984) Monoclonal antibodies specific for glial and neuronal antigens in the young rat cerebellum.Neuroscience Letters 51, 119–25.
GIOTTA, G. J., HEITZMANN, J. G., DUBOIS, M. & BOWMAN, P. D. (1985) Presence of a novel epithelial antigen on rat cerebellar cell lines as detected by a monoclonal antibody.Developmental Brain Research 21, 61–71.
GOLDOWITZ, D. & MULLEN, R. J. (1982) Granule cell as a site of gene action in the weaver mouse cerebellum: evidence from heterozygous mutant chimeras.Journal of Neuroscience 2, 1474–85.
HATTEN, M. E. & LIEM, R. K. H. (1981) Astroglial cells provide a template for the positioning of developing cerebellar neuronsin vitro.Journal of Cell Biology 90, 622–30.
HATTEN, M. E., LIEM, R. K. H. & MASON, C. A. (1986) Weaver mouse cerebellar granule neurons fail to migrate on wild-type astroglial processesin vitro.Journal of Neuroscience 6, 2676–83.
HERRUP, K. & TRENKNER, E. (1987) Regional differences in cytoarchitecture of the weaver cerebellum suggests a new model for weaver gene action.Neuroscience 23, 871–85.
HOCKFIELD, S. (1987) A Mab to a unique cerebellar neuron generated by immunosuppression and rapid immunization.Science 237, 67–70.
HOFFMAN, S., CROSSIN, K. L. & EDELMAN, G. M. (1988) Molecular forms, binding functions, and developmental expression of cytotactin and cytotactin-binding proteoglycan, an interactive pair of extracellular matrix molecules.Journal of Cell Biology 106, 519–32.
KOHLER, G. & MILSTEIN, C. (1975) Continuous cultures of fused cells secreting antibody of predefined specificity.Nature 256, 495–7.
LAEMMLI, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature 227, 680–5.
LANGLEY, O. K., FOUCAUD, B., GHANDOUR, M. S., DE BARRY, J., SCHLADENHAUFEN, Y. & GOMBOS, G. (1985) A developmentally modified neuron specific marker in rodent cerebellum.Neuroscience 14, 147–57.
LETOURNEAU, P. C. (1982) Nerve fibre growth and its regulation by extrinsic factors. InNeuronal Development (edited by SPITZER, N. C.), pp. 213–54. New York: Plenum Press.
LINDNER, J., RATHJEN, F. G. & SCHACHNER, M. (1983) L1 and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum.Nature 305, 427–30.
MENDEZ-OTERO, R., SCHLOSSHAUER, B., BARNSTABLE, C. J. & CONSTANTINE-PATON, M. (1988) A developmentally regulated antigen associated with neural cell and process migration.Journal of Neuroscience 8, 564–79.
PALAY, S. L. & CHAN-PALAY, V. (1974)Cerebellar Cortex. Berlin: Springer-Verlag.
PIGOTT, R. & KELLY, J. S. (1986) Immunocytochemical and biochemical studies with the monoclonal antibody 69A1: similarities of the antigen with cell adhesion molecules L1, Nile and Ng-CAM.Developmental Brain Research 29, 111–22.
RAKIC, P. (1985) Mechanisms of neuronal migration in developing cerebellar cortex. InMolecular Mechanisms of Neural Development (edited by EDELMAN, G. M., GALL, W. E. & COWAN, W. M.), pp. 139–60. New York: John Wiley & Sons.
RAKIC, P. & SIDMAN, R. L. (1973a) Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice.Journal of Comparative Neurology 152, 103–32.
RAKIC, P. & SIDMAN, R. L. (1973b) Organization of cerebellar cortex secondary to deficit in weaver mutant mice.Journal of Comparative Neurology 152, 133–62.
RAKIC, P. & SIDMAN, R. L. (1973c) Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia.Proceedings of the National Academy of Sciences USA 70, 240–4.
RATHJEN, F. G. & SCHACHNER, M. (1984) Immunocytological and biochemical characterization of a new neuronal surface component which is involved in cell adhesion.EMBO Journal 3, 1–10.
RIEDERER, B. M., ZAGON, I. S. & GOODMAN, S. R. (1987) Brain spectrin (240/235) and brain spectrin (240/235E): differential expression during mouse brain development.Journal of Neuroscience 7, 864–74.
ROUSE, R. V., BOLIN, L. M., BENDER, J. R. & KYEWSKI, B. A. (1988) Monoclonal antibodies reactive with subsets of mouse and human thymic epithelial cells.Journal of Histochemistry and Cytochemistry 36, 1511–17.
RUTISHAUSER, U. & JESSELL, T. M. (1988) Cell adhesion molecules in vertebrate neural development.Physiological Reviews 68, 819–57.
SANES, J. (1983) Roles of extracellular matrix in neural development.Annual Reviews of Physiology 45, 581–600.
SCHACHNER, M. (1982) Immunological analysis of cellular heterogeneity in the cerebellum. InNeuroimmunology (edited by BROCKES, J.), pp. 215–50. New York: Plenum Press.
SCHACHNER, M., FAISSNER, A., KRUSE, J., LINDNER, J., MEIER, D. H., RATHJEN, F. G. & WERNECKE, H. (1983) Cell-type specificity and developmental expression of neural cell—surface components involved in cell interactions and of structurally related molecules.Cold Spring Harbor Symposia on Quantitative Biology 48, 557–68.
SCHWOB, J. E. & PRICE, J. L. (1984) The development of axonal connections in the central olfactory system of rats.Journal of Comparative Neurology 223, 177–202.
SELAK, I., FOIDART, J. M. & MOONEN, G. (1985) Laminin promotes cerebellar granule cell migrationin vitro and is synthesized by cultured astrocytes.Developmental Neuroscience 7, 278–85.
SIDMAN, R. L., WILLINGER, M. & MARGOLIS, D. M. (1983) Mouse weaver mutation affects granule cell neurite growthin vitro.Birth Defects: Original Article Series 19:4, 189–200.
SILVER, J., LORENZ, S. E., WAHLSTEN, D. & COUGHLIN, J. (1982) Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies,in vivo, on the role of preformed glial pathways.Journal of Comparative Neurology 210, 10–19.
SMEYNE, R. J. & GOLDOWITZ, D. (1989) Development and death of external granular layer cells in the weaver mouse cerebellum: a quantitative study.Journal of Neuroscience 9, 1608–20.
SOTELO, C. & CHANGEAUX, J.-P. (1974) Bergmann fibres and granular cell migration in the cerebellum of homozygous weaver mutant mouse.Brain Research 77, 484–91.
STREETER, P. S., ROUSE, B. T. N. & BUTCHER, E. C. (1988) Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes.Journal of Cell Biology 107, 1853–62.
TOWBIN, H. T., STAEHELIN, T. & GORDON, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets; procedure and some applications.Proceedings of the National Academy of Sciences USA 76, 4350–4.
TRENKNER, E., SMITH, D. & SEGIL, N. (1984) Is cerebellar granule cell migration regulated by an internal clock?Journal of Neuroscience 4, 2850–5.
WILLINGER, M. & MARGOLIS, D. M. (1985) Effect of the weaver mutation on cerebellar neuron differentiation. II. Quantitation of neuron behaviour in culture.Developmental Biology 107, 173–9.
WILLINGER, M., MARGOLIS, D. M. & SIDMAN, R. R. (1981) Neuronal differentiation in cultures of weaver (wv) mutant mouse cerebellum.Journal of Supramolecular Structure and Cellular Biochemistry 17, 79–86.
YAMAMOTO, M., BOYER, A. M., CRANDALL, J. E., EDWARDS, M. & TANAKA, H. (1986) Distribution of stage-specific neurite-associated proteins in the developing murine nervous system recognized by a monoclonal antibody.Journal of Neuroscience 6, 3576–94.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pickford, L.B., Mayer, D.N., Bolin, L.M. et al. Transiently expressed, neural-specific molecule associated with premigratory granule cells in postnatal mouse cerebellum. J Neurocytol 18, 465–478 (1989). https://doi.org/10.1007/BF01474543
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01474543