Abstract
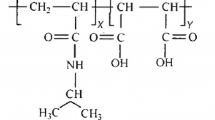
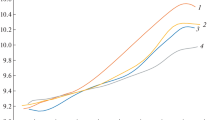
The titration curves of latex dispersions of ethyl acrylate — methacrylic acid copolymers have a rather complex shape which indicates a strong dependence of the apparent dissociation constant of carboxylic groups on the degree of neutralization and copolymer composition. These dependences seem to be related to changes in the macroscopic structure (swelling and disintegration) of dispersion particles during alkalization.
Similar content being viewed by others
References
Fordyce DB, Dupre J, Toy W (1959) Official Digest 2:284
Verbrugge CJ (1970) J Appl Polym Sci 14:897
Šňupárek J, KrŠka F (1976) J Appl Polym Sci 20:1753
Šňupárek J (1985) Macromal Chem, Suppl 10/11:129
Morgan LW, Jensen DP (1985) Macromol Chem, Suppl 10/11:59
Gregor HP, Frederick M (1957) J Polym Sci 23:451
Quadrat O, Mrkvičková L, Jasná E, Šňupárek J Colloid Polym Sci in press
Prokopová E, Štol M, KniŽáková E, Bohdanecký M (1979) Makromol Chem 180:615
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Quadrat, O., Mrkvičková, L., Jasná, E. et al. Characteristic changes of pH during the alkalization of latex dispersions of the ethyl acrylate — methacrylic acid copolymers. Colloid & Polymer Sci 268, 921–923 (1990). https://doi.org/10.1007/BF01469370
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01469370