Abstract
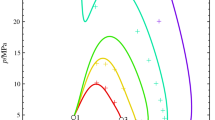
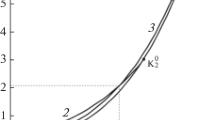
An algorithm has been developed for calculating the gas-gas critical line of type-III binary fluid mixtures for extended corresponding-states (ECS) models. The algorithm searches for an extremum in pressure on the spinodal curve of an isothermal pressure-composition phase diagram of a binary mixture. The method has been applied to solutions of carbon dioxide and of nitrogen in water. starting at the water critical point. Two variants of ECS have been tested for their ability to represent reliablePVTx data in the nitrogen-water mixture. It is demonstrated that in the latter system both ECS variants produce an artifact in the gas-gas critical line in the range of 0–0.2 mole fraction of nitrogen.
Similar content being viewed by others
References
P. H. Van Konynenburg and R. L. Scott.Phil. Trans. Roy Soc. 98: 48 (1980).
NIST Thermophysical Properties of Hydrocarbon Mixtures Database, SUPERTRAPP. Version 1.04 (Natl. Inst. Stand. Tech., Gaithersburg, MD, 1992).
NIST Mixture Property Program. NIST14, Version 9.08 (Natl. Inst. Stand. Tech., Gaithersburg. MD. 1993).
K. D. Romig. Jr.. and H. J. M. Hanley.Cryogenics 26: 33 (1986): 29:65 (1989).
A. Fenghour. W. A. Wakeham. D. Ferguson, A. C. Scott, and J. T. R. Watson,J. Chem. Thermodyn. 25: 831 (1993).
J. S. Gallagher, R. Crovetto, and J. M. H. Levelt Sengers.J. Phys. Chem. Ref. Data 22: 431 (1993).
J. S. Gallagher, J. M. H. Levelt Sengers, I. M. Abdulagatov. J. T. R. Watson, and A. Fenghour. NIST Teelt. Note 1404 (U.S. Government Printing Office. Washington, DC, 1993).
L. Haar, J. S. Gallagher, and G. S. Kell,NBS'NRC Steam Tables (Hemisphere. Washington, DC. 1984).
A. van Pelt. C. J. Peters, and J. de Swaan Arons,J. Chem. Phys. 95: 7569 (1991).
D. G. Friend and J. F. Ely.Fluid Phase Equil.79: 77 (1992).
I. M. Abdulagatov, A. R. Bazacv, and A. E. Ramazanova.Int. J. Thermophys. 14: 231 (1993).
K. Tödheide and E. U. Franck,Z. Phys. Chem. N.F 37: 387 (1963).
M. L. Lapas and E. U. Franck.Ber. Bunsenges. Phys. Chem. 89: 793 (1985).
S. Takenouchi and G. C. Kennedy,Am. J. Sci. 262: 1055 (1964).
A. E. Mather and E. U. Franck,J. Phys. Chem. 96: 6 (1992).
D. S. Tsiklis and N. Ya. Maslennikova,Dokl. Akad. Nauk. SSSR 161: 645 (1965): V. M. Prokhorov and D. S. Tsiklis. Russ. J. Phys. Chem. 44:1173 (1970).
B. E. Eaton, J. Stecki, P. Wielopolski, and H. J. M. Hanley,J. Res. Natl. Bur. Stand. USA 86: 419 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gallagher, J.S., Friend, D.G., Given, J.A. et al. Critical lines for type-III aqueous mixtures by generalized corresponding-states models. Int J Thermophys 15, 1271–1278 (1994). https://doi.org/10.1007/BF01458835
Issue Date:
DOI: https://doi.org/10.1007/BF01458835