Abstract
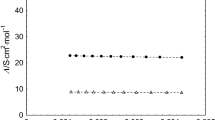
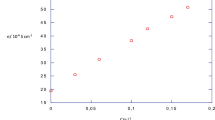
The results of conductivity measurements for aqueous solutions of poly(1,3-propylene phosphate) (PPP), which can be considered as a synthetic analogue of naturally occurring teichoic acids, are reported. Experiments were carried out with oligomeric fractions of a polymer in acidic, sodium, potassium, magnesium and calcium forms. The concentration and molecular weight dependence of the equivalent conductivity of PPP was analysed and the limiting equivalent conductivity determined. From the conductivity data, the polyion-counterion interaction parameter F and the equivalent conductivity of a polyion λ p were calculated. It was shown that both F and λ p depend on polyelectrolyte solution concentration and molecular weight of PPP. Conclusions concerning mono- and divalent metal ions binding to PPP are drawn.
Similar content being viewed by others
References
Heprinstall S, Archibald AR, Baddiley J (1970) Nature 225:519
Hughes AH, Hancock IC, Baddiley J (1973) Biochem J 132:83
Lambert PA, Hancock IC, Baddiley J (1975) Biochem J 149:519
Munson RS, Glaser L (1981) In: Ginsburg V (ed) Biology of Carbohydrates, Vol I, Wiley, New York, p 109
Naumova IB (1973) Usp Sovrem Biol 75:357
Wódzki R, Kałuzyński K (1984) Makromol Chem Rapid Commun 5:385
Litowska M, Narebska A (1984) Coll Polym Sci 262:455, 461
Litowska M (1986) Coll Polym Sci 264:352
Huizinga JR, Grieger PF, Wall FT (1950) J Am Chem Soc 72:4228
Darskus RL, Jordan DO, Kurucsev T (1966) Trans Faraday Soc 62:2876
Jordan DO, Kurucsev T, Martin M (1969) Trans Faraday Soc 65:606
Kowblanski M, Ander P (1977) J Phys Chem 81:2024
Manning GS (1970) Biopolymer 9:1534
Dolar D, Špan J, Pretnar A (1968) J Polym Sci, Part C 16:3557
Katchalsky A (1971) Pure Appl Chem 26:327
Špan J, Gačeša A (1974) Z Phys Chem 90:26
Joshi YM, Kwak JCT (1980) Biophys Chem 12:323
Thibault JS, Rinaudo M (1985) Biopolymers 24:2131
Vink H (1982) Makromol Chem 183:2273
Vink H (1983) J Chem Soc Faraday Trans 1 79:1403
Kałużyński K, Libiszowski J, Penczek S (1976) Macromolecules 9:365
Szymczak J, Holyk P, Ander P (1975) J Phys Chem 79:269
Kwak JCT, Hayes PC (1975) J Phys Chem 79:265
Eisenberg H, Ram Mohan G (1959) J Phys Chem 63:671
Chatterji AC, Bhargara HH (1960) Kolloid-Z 170:116
Eisenberg H (1958) J Polym Sci 30:47
Varoqui R, Strauss UP (1968) J Phys Chem 72:2507
Nelson RE, Ander P (1971) J Phys Chem 75:1691
Strauss UP, Ross PD (1959) J Am Chem Soc 81:5295
Kennedy J, Wheeler VJ (1959) Chem Ind 1577
Kuznetsov IA, Gorshkov VI, Ivanov VA, Kargov SI, Korolev NI, Filippov SM, Khamishov RKh (1984) React Polym 3:37
Wódzki R, Narebska A (1986) In: Kuczera J, Przestalski S (eds) Biophysics of Membrane Transport, Vol II, Agricultural University of Wroław, p 324
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ostrowska-Czubenko, J., Wódzki, R. Electrolytic conductivity of poly(1,3-propylene phosphate) solutions containing mono- and divalent-counterions. Colloid & Polymer Sci 266, 35–40 (1988). https://doi.org/10.1007/BF01451529
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01451529