Abstract
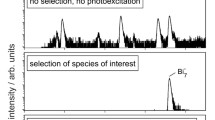
Kinetic studies of the reactions of neutral lead clusters with NO2, NO and O2 were performed at 300 K. Reaction with NO2 is rapid, with the observed second-order rate constants for most clusters being between 0.2 and 5 × 10−11 cm3/s. There is a general trend of increasing rate with cluster size, although a few clusters display unusually high or low rates compared to ones of neighboring size. The reactions with NO are considerably slower by factors ranging from about 5 to 10. Reaction products are observed by laser ionization at 193 and 222 nm in conjunction with time-of-flight mass spectrometry. At lower fluence, the association products Pb x (NO2)+ and Pb x (NO2) +2 are observed in the case of reactions with NO2. At higher laser fluence, Pb + x and Pb x O −1+ x dominate the mass spectra of Pb x reactions with NO2, showing that the products fragment to more stable oxides. No reaction with oxygen was observed for any cluster, setting upper limits on the rates of 5 × 10−14 cm3/s.
Similar content being viewed by others
References
Morse, M.D., Geusic, M.E., Heath, J.R., Smalley, R.E.: J. Chem. Phys.83, 2293 (1985); Whetten, R.L., Cox, D.M., Trevor, D.J., Kaldor, A.: Phys. Rev. Lett.54, 1494 (1985); Kaldor, A. Cox, D.M., Zakin, M.R.: In: Advances in chemical physics, vol. LXX, Prigogine, I., Rice, S.A. (eds.), 211 (1988). Jarrold, M.F.: In: Gas phase inorganic chemistry. Russell, D.H. (ed.), Chap. 5, p. 137. New York: Plenum Press 1989; Riley, S.J.: J. Chem. Phys.86, 1066 (1987). Martin, T.P., Bergmann, T., Göhlich, H., Lang, T.: Z. Phys. D19, 25 (1991)
Sattler, K.: Z. Phys. D3, 223 (1986); Wheeler, R.G., Lai Hing, K., Wilson, W.L., Duncan, M.A.: J. Chem. Phys.88, 2831 (1988); Schild, D., Pflaum, R., Sattler, K., Recknagel, E.: Proc. Int. Symp. Rarefield Gas Dyn., 15th, 79 (1986)
Antonio, G.A., Feuston, B.P., Kalia, R.K., Vashishta, P.: J. Chem. Phys.88, 7671 (1988); Heath, J.R., Liu, Y., O'Brien, S.C., Zhang, Q-L., Curl, R.F., Tittel, F.K., Smalley, R.E.: J. Chem. Phys.83, 5520 (1985)
Goldenfeld, I., Frank, F., Schultze, W., Winter, B.: Int. J. Mass Spec. Ion Proc.71, 103 (1986)
Pacyna, J.M.: In: Toxic metals in the atmosphere. Nriagu, J.O., Davidson, C.I. (eds.). New York: Wiley 1986
Chadwick, D., Christie, A.B.: J. Chem. Soc. Faraday Trans. 276, 267 (1980)
Hewitt, R.W., Winograd, N.: Surf. Scie.78, 1 (1978); Eldridge, J.M., Dong, D.W.: Surf. Sci.40, 512 (1973); Macmillan-Jones, J.G., Londry, F.A., Slavin, A.J.: Surf. Sci.186, 375 (1987); Kim, K.S., Winograd, N.:19, 209 (1973)
Brown, H.E.: In: Lead oxide, properties and applications. International Lead Zinc Research Organization, Inc., 1985
Joyner, R.W., Kishi, K., Robert, M.W.: Proc. R. Soc. London A60, 1865 (1985)
Saleh, J.M., Wells, B.R., Roberts, M.W.: Trans. Faraday Soc.60, 1865 (1985)
Evans, S., Thomas, J.M.: J. Chem. Soc. Faraday Trans. 271, 313 (1974)
Farley, R.W.: Ph.D. Thesis, University of Colorado, Boulder, (1989)
Duthler, C.J., Johnson, S.E., Broida, H.P.: Phys. Rev. Lett.26, 1236 (1971)
Hayashi, C.: Phys. Today40, 44 (1987)
Farley, R.W., Ziemann, P., Castleman, A.W. Jr.: Z. Phys. D14, 353 (1989)
Upschulte, B.L.: Ph. D. thesis, University of Colorado, Boulder (1986)
Philipps, J.C.: Chem. Rev.86, 619 (1986)
Peterson, K.I., Dao, P.D., Castleman, A.W. Jr.: J. Chem. Phys.80, 1780 (1984); Peterson, K.I., Dao, P.D., Castleman, A.W. Jr.: J. Chem. Phys.79, 777 (1983)
Sattler, K., Mühlbach, J., Echt, O., Pfau, P., Recknagel, E.: Phys. Rev. Lett.47, 160 (1981)
Pfau, P., Sattler, K., Pflaum, R., Recknagel, E.: Phys. Lett.104A, 262 (1984)
Goldenfeld, I., Frank, F., Schulze, W., Winter, B.: Int. J. Mass. Spectrum. Ion Proc.71, 103 (1986)
Drowart, J., Colin, R., Exsteen, G.: Trans. Faraday Soc.61, 1376 (1965)
Ogden, J.S., Ricks, M.J.: J. Chem. Phys.56, 1658 (1970)
Novinsky, H.J., Pflaum, R., Pfau, P., Sattler, K., Recknagel, E.: Surf. Sci.156, 126 (1985)
Drowart, J., Colin, R., Exsteen, G.: Trans. Faraday Soc.61, 1376 (1985)
Ogden, J.S., Ricks, M.J.: J. Chem. Phys.56, 1658 (1970)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Farley, R.W., Ziemann, P.J., Keesee, R.G. et al. Kinetic study of neutral lead cluster reactions. Z Phys D - Atoms, Molecules and Clusters 25, 267–273 (1993). https://doi.org/10.1007/BF01426890
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01426890