Abstract
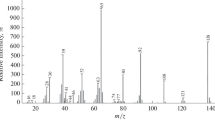
Photodissociation of negatively charged sulfur dioxide clusters (SO2) − n , 2≦n≦11, was investigated in the wavelength range between 458 nm and 600 nm using a tandem mass spectrometer. The spectral position of the absorption band remains unchanged, however it exhibits narrowing with increasing cluster size. The smooth evolution of the spectra shows that the clusters are composed of a dimer anion core surrounded by neutral molecules. The analysis of the fragmentation products reveals that the absorption of a photon is followed by statistical evaporation of neutrals with a mean energy loss of 0.28±0.05 eV per evaporated monomer in the large cluster limit.
Similar content being viewed by others
References
Hodges, R.V., Lee, L.C., Moseley, J.T.: J. Chem. Phys.72, 2998 (1980)
Smith, G.P., Cosby, P.C., Moseley, J.T.: J. Chem. Phys.67, 3818 (1977)
Keesee, R.G., Castleman Jr., A.W.: Structure of solvated cluster ions, Maier, J.P. (ed.), Ion and cluster ion spectroscopy and structure, Amsterdam: Elsevier 1989
McDaniel, E.W., Mason, E.A.: The mobility and diffusion of ions in gases, New York: Wiley 1973
Keesee, R.G., Lee, N., Castleman Jr., A.W.: J. Chem. Phys.73, 2195 (1980)
Breen, J.J., Kilgore, K., Stephan, K., Hofmann-Sievert, R., Kay, B.D., Keesee, R.G., Märk, T.D., Castleman Jr., A.W.: Chem. Phys.91, 305 (1984)
Nimlos, M.R., Ellison, G.B.: J. Phys. Chem.90, 2574 (1986)
Bowen, K.H., Liesegang, G.W., Sanders, R.A., Herschbach, D.R.: J. Phys. Chem.87, 557 (1983)
Snodgrass, J.T., Coe, J.V., Freidhoff, C.B., McHugh, K.M., Bowen, K.H.: J. Chem. Phys.88, 8014 (1988)
Dinse, K.P., Möbius, K.: Z. Naturforsch.23a, 695 (1968)
Rinker, R.G., Lynn, S.: J. Phys. Chem.72, 4706 (1968)
Magno, F., Mazzocchin, G.A., Bontempelli, G.: Electroanal. chem. interfacial electrochemistry57, 89 (1974)
Martin, R.P., Sawyer, D.: Inorg. Chem.11, 2644 (1972)
Ito, O., Kuwashima, S., Matsuda, M.: Bull. Chem. Soc. Jap.49, 327 (1976)
Hodges, R.V., Vanderhoff, J.A.: J. Chem. Phys.72, 3517 (1980)
Castleman Jr., A.W., Albertoni, C.R., Marti, K., Hunton, D.E., Keesee, R.G.: Faraday Discuss. Chem. Soc.82, 262 (1986)
Kim, H.S., Bowers, M.T.: J. Chem. Phys.85, 2718 (1986)
Märk, T.D., Scheier, P., Stamatovic, A.: Chem. Phys. Lett.136, 177 (1987)
Stamatovic, A., Scheier, P., Märk, T.D.: Z. Phys. D — Atoms, Molecules and Clusters6, 351 (1987)
Heil, H.: Z. Phys.120, 212 (1943)
Ardenne von, M.: Phys. Z.43, 91 (1942)
Wåhlin, L.: Nucl. Instrum. Methods27, 55 (1964)
Harting, E., Read, F.H.: Electrostatic lenses, Amsterdam: Elsevier 1976
Dahl, D.A., Delmore, J.E.: SIMION, Idaho national engineering laboratory EG&G Idaho Inc., Idaho Falls, 1988
Zeman, H.D.: Rev. Sci. Instrum.48, 1079 (1977)
Farley, J.W.: Rev. Sci. Instrum.56, 1834 (1985)
Elmore, W.C., W. Garret, M.: Rev. Sci. Instrum.25, 480 (1954)
Dayton, I.E., Shoemaker, F.C., Mozley, R.F.: Rev. Sci. Instrum.25, 485 (1954)
Branscomb, L.M., Smith, S.J., Tisone, G.: J. Chem. Phys.43, 2906 (1965)
Feldmann, D.: Z. Naturforsch.25, 621 (1970)
Dresch, T., Kramer, H., Thurner, Y., Weber, R.: Chem. Phys. Lett.,177, 383 (1991)
Sullivan, S.A., Freiser, B.S., Beauchamp, J.L.: Chem. Phys. Lett.48, 294 (1977)
Alexander, M.L., Johnson, M.A., Levinger, N.E., Lineberger, W.C.: Phys. Rev. Lett.57, 976 (1986)
Alexander, M.L., Johnson, M.A., Lineberger, W.C.: J. Chem. Phys.82, 5288 (1985)
Engelking, P.C.: J. Chem. Phys.87, 936 (1987)
Engelking, P.C.: J. Chem. Phys.85, 3103 (1986)
CRC handbook of physics and chemistry, Weast, R.C. (ed.). Cleveland: Chemical Rubber 1977
Herzberg, G.: Molecular spectra and molecular structure. Vol. 1. New York: Van Nostrand 1950
Johnson, M.A., Alexander, M.L., Lineberger, W.C.: Chem. Phys. Lett.112, 285 (1984)
Illies, A.J., Jarrold, M.F., Wagner-Redeker, W., Bowers, M.T.: J. Phys. Chem.88, 5204 (1984)
Klots, C.E.: J. Chem. Phys.83, 5854 (1985)
Klots, C.E.: Z. Phys. D — Atoms, Molecules and Clusters5, 83 (1987)
Klots, C.E.: J. Phys. Chem.92, 5864 (1988)
Ray, D., Levinger, N.E., Papanikolas, J.M., Lineberger, W.C.: J. Chem. Phys.91, 6533 (1989)
Kim, H.S., Jarrold, M.F., Bowers, M.T.: J. Chem. Phys.84, 4882 (1986)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dresch, T., Kramer, H., Thurner, Y. et al. Photodissociation of sulfur dioxide cluster anions. Z Phys D - Atoms, Molecules and Clusters 18, 391–397 (1991). https://doi.org/10.1007/BF01426603
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01426603