Summary
-
1.
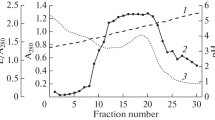
Prekallikrein, prepared according to Nagasawaet al., has been purified further by agarose gel electrophoresis. It can be activated directly by Hageman factor and by solid phase trypsin. Its specific activity (benzoyl arginine ethyl ester as substrate) was 13.2 μM min−1 mg−1. Gel filtration on a calibrated column of sephadex G 200 revealed a molecular weight of 101,000, which is close to the value reported for casein-activated kallikrein.
-
2.
The contact-activated bovine enzyme was very similar to casein-activated porcine kallikrein. Both enzymes hydrolyzed benzoyl arginine ethyl ester and tosylarginine methyl ester with about the same speed. The sensitivity of the two preparations against trasylol®, soy bean inhibitor, and serum inhibitor was also not different.
-
3.
In contrast to previous assumptions, purified bovine LMW kininogen (MW 50,000) proved to be substrate for contact-activated kallikrein. As with the caseinactivated enzyme, the total bradykinin content of the kininogen could be released.
-
4.
Serum, even when heated previously to 61°C, contains inhibitors against kallikreins activated by contact or casein, as well as against trypsin. Whereas pretreatment according to Diniz and Carvalho (pH about 2; 98°C) renders the serum more susceptible to trypsin, such denaturated substrate is less accessible for both kallikreins. Serum or plasma pretreated according to Horton (pH2; 37°C), yield at least 50% of the kinin activity obtained with trypsin, irrespective of the kallikreins used.
-
5.
When acid-treated (according to Horton) 61°-serum has been incubated first with casein-activated kallikrein, contact-activated enzyme does not release additional kinin activity and vice versa.
-
6.
Plasma which has been rotated exhaustively with glass, nevertheless contains substrate for contact-activated kallikrein.
-
7.
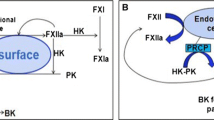
Addition of Hageman factor to Horton's substrate does not increase the kinin yield above that obtained by glass-contact of normal plasma. Addition of prekallikrein, however, increases the kinin yield about threefold. Therefore, neither Hageman factor nor substrate, but the actual amount of active kallikrein limits the kinin yield upon glass activation.
-
8.
Our experiments with serum kallikreins and their substrates can be interpreted without assuming a distinct, contact activated kinin system.
Similar content being viewed by others
References
Aarsen, P. N.: Sensitization of guinea-pig ileum to the action of bradykinin by trypsin hydrolysate of ox and rabbit plasma. Brit. J. Pharmacol.32, 453–465 (1968).
Andrews, P.: The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem. J.96, 595–606 (1965).
Arndts, D.: Radioimmunologische Ultramikrobestimmungen von Proteinen und Polypeptiden: Entwicklung, Vergleich und Anwendungsmöglichkeiten. Inaug.- Diss., Gießen 1968.
Colman, R., Mattler, M., Sherry, S.: Studies on prekallikrein (kallikreinogen)-kallikrein enzyme system of human plasma. I. Isolation and purification of plasma kallikreins. II. Evidence relating the koalin-activated arginine esterase to plasma kallikrein. J. clin. Invest.48, 11–32 (1969).
Diniz, C. R., Carvalho, J. E.: A micro method for determination of bradykininogen under several conditions. Ann. N. Y. Acad. Sci.104, 77–89 (1963).
Eisen, V.: Observations on intrinsic kinin-forming factors in human plasma: the effect of acid, acetone, chloroform, heat and euglobulin separation on kinin formation. J. Physiol. (Lond.)166, 496–513 (1963).
—: Enzymic aspects of plasma kinin formation. Proc. roy. Soc. B173, 351–359 (1969).
—, Glanville, K. L. A.: Separation of kinin-forming factors in human plasma. Brit. J. exp. Path.50, 427–437 (1969).
—, Keele, C. A.: Intrinsic plasma kinin forming factors in human plasma. J. Physiol. (Lond.)154, 20P-22P (1960).
—, Vogt, W.: Plasma kininogenases and their activators. In: Handbook of Experimental Pharmacology, vol 25, pp. 82–130. Berlin-Heidelberg-New York: Springer 1970.
Habermann, E.: Trennung und Reinigung von Pankreaskallikreinen, Hoppe Seylers Z. physiol. Chem.328, 15–23 (1962).
—: Über pH-bedingte Modifikationen des kininliefernden α-Globulins (Kininogen) aus Rinderserum und das Molekulargewicht von Kininogen I. Biochem. Z.337, 440–448 (1963).
—: Inhibitors of kallikrein and other proteinases from animal blood. Ann. N. Y. Acad. Sci.146, 479–490 (1968).
—: Kininogens. In: Handbook of Experimental Pharmacology, vol. 25, pp. 250 to 288. Berlin-Heidelberg-New York: Springer 1970.
—, Blennemann, G.: Über Substrate und Reaktionsprodukte der kininbildenden Enzyme Trypsin, Serum- und Pankreaskallikrein sowie von Crotalusgift. Naunyn-Schmiedebergs Arch. exp. Path. Pharmak.249, 357–373 (1964).
—, Jahrreiss, R.: On the endogenous mechanism of kinin release. II. Attempts at discriminating kininogens. Naunyn-Schmiedebergs Arch. Pharmak.269, 101 to 111 (1971).
—, Klett, W.: Reinigung und einige Eigenschaften eines Kallikreins aus Schweineserum. Biochem. Z.346, 133–158 (1966).
Habermann, E., Klett, W., Rosenbusch, G.: Partielle Reinigung und einige Eigenschaften eines Kininogens aus Rinderblut. Hoppe Seylers Z. physiol.Chem.332, 121–142 (1963).
Hamberg, U., Elg, P., Stelwagen, P.: Tryptic and plasmic peptide fragments increasing the effect of bradykinin on isolated smooth muscle. Scand. J. clin. Lab. Invest. (Suppl.)24, 21–50 (1969).
Henriques, O. B., Stolpnik, V., Kusnetsova, V., Astrakan, M.: Glass-activated plasma kallikrein. Biochem. Pharmacol.19, 2915–2921 (1970).
Horton, E. W.: The assay of kallikrein in urine. J. Physiol. (Lond.)142, 36P (1958).
Jacobsen, S., Kriz, M.: Some data on two purified kininogens from human plasma. Brit. J. Pharmacol.29, 25–36 (1967).
Jahrreiss, R., Habermann, E.: Isolierung und Struktur peptischer kininliefernder Peptide aus Rinderserum. Naunyn-Schmiedebergs Arch. Pharmak.264, 172 to 186 (1969).
— —: In vitro investigations on some components of the kinin system (kininogen, serum kallikrein, Hageman factor) and their interaction. In F. Sicuteri (Ed): Bradykinin and related kinins: Cardiovascular, biochemical, and neural actions. New York-London: Plenum Press 1970.
Kagen, L. J.: Some biochemical and biophysical properties of the human permeability globulins. Brit. J. exp. Path.45, 604 (1964).
Kellermeyer, W. F., Kellermeyer, R. W.: Hageman factor activation and kinin formation in human plasma induced by cellulose-sulfate solutions. Proc. Soc. exp. Biol. (N. Y.)130, 1310–1314 (1969).
Laufer, A. L., Gulikova, O. M., Paskhina, T. S.: Two kinin-producing enzymes from the blood plasma of man. Biokhimiya34, 3–12 (1969).
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with the folin reagent. J. biol. Chem.193, 265–275 (1951).
Margolis, J.: Activation of plasma by contact with glass: evidence for a common reaction which releases plasma kinin and initiates coagulation. J. Physiol. (Lond.)144, 1–22 (1958).
—: Activation of Hageman factor by saturated fatty acids. Aust. J. exp. Biol.40, 505–514 (1962).
—: Interrelationship of coagulation of plasma and release of peptides. Ann. N. Y. Acad. Sci.104, 133–145 (1963).
—, Bishop, E. A.: Studies on plasma kinins. I. The composition of kininogen complex. Aust. J. exp. Biol.41, 293–306 (1963).
Movat, H. Z., DiLorenzo, N. L., Treloar, M. P.: Activation of the plasma kinin system by antigen-antibody aggregates. II. Isolation of permeability-enhancing and kinin-releasing fractions from activated guinea pig serum. Lab. Invest.19, 201–210 (1968).
- Poon, M.-Ch., Takeuchi, Y.: The kinin-system of human plasma. I. Isolation of a low molecular weight activator of prekallikrein. (In press.)
Nagasawa, S., Takahashi, H., Koida, M., Suzuki, T., Schoenmakers, J. G. G.: Partial purification of bovine plasma kallikreinogen, its activation by the Hageman factor. Biochem. biophys. Res. Commun.32, 644–649 (1968).
Ratnoff, O. D.: Activation of Hageman factor by L-Homocystine. Science162, 1007–1009 (1968).
—, Crum, J. D.: Activation of Hageman factor by solutions of ellagic acid. J. Lab. clin. Med.63, 359–377 (1964).
Roberts, P. S.: Measurement of the rate of plasmin action on synthetic substrates. J. biol. Chem.232, 285–291 (1958).
Schoenmakers, J. G. G., Matze, R., Haanen, C., Zilliken, F.: Hageman factor, a novel sialoglycoprotein with esterase activity. Biochim. biophys. Acta (Amst.)101, 166–176 (1963).
Schwert, G. W., Takenaka, Y.: A spectrophotometric determination of trypsin and chymotrypsin. Biochim. biophys. Acta (Amst.)16, 570–575 (1955).
Seidel, G., Vogt, W.: Kininogenspezifität von acetonaktiviertem menschlichem Plasmakallikrein. Naunyn-Schmiedebergs Arch. Pharmak. exp. Path.262, 135–138 (1969).
Suzuki, T., Iwanaga, S., Nagasawa, S., Sato, T.: Purification and properties of bradykininogen and of the bradykinin-releasing and destroying enzymes in snake venom. In: E. G. Erdös, N. Back, and F. Sicuteri: Hypotensive peptides pp. 149–160. Berlin-Heidelberg-New York: Springer 1966.
Temme, H., Jahrreiss, R., Habermann, E., Zilliken, F.: Aktivierung von Gerinnungs- und Kininsystem durch eine Plasmaesterase (Hageman-Faktor). Reinigung und Wirkungsbedingungen. Hoppe Seylers Z. physiol. Chem.350, 519–521 (1969).
Vogt, W., Garbe, G., Schmidt, G.: Untersuchungen zur Existenz zweier verschiedener kininbildender Systeme in menschlichem Plasma. Naunyn-Schmiedebergs Arch. Pharmak. exp. Path.256, 127–138 (1967).
—, Wawretschek, W.: Weitere Untersuchungen zur Existenz zweier kininbildender Systeme in menschlichem Plasma. Naunyn-Schmiedebergs Arch. Pharmak. exp. Path.260, 223–230 (1968).
Webster, M. E.: Human plasma kallikrein, its activation and pathological role. Fed. Proc.27, 84–89 (1968).
—, Ratnoff, O. D.: Role of Hageman factor in the activation of vasodilator activity in human plasma. Nature (Lond.)192, 180–181 (1961).
Wilner, G. D., Nossel, H. L., LeRoy, E. C.: Activation of Hageman factor by collagen. J. clin. Invest.47, 2608–2615 (1968).
Wuepper, K. D., Cochrane, C. G.: Plasma prokininogenase: mechanism of activation. Fed. Proc.29, 811 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jahrreiss, R., Habermann, E. On the endogenous mechanism of kinin release. Naunyn-Schmiedebergs Arch. Pharmak. 269, 85–100 (1971). https://doi.org/10.1007/BF01422018
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01422018