Summary
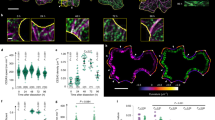
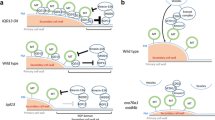
Cortical microtubules (MTs) have been implicated in the morphogenesis of plant cells by regulating the orientation of newly deposited cellulose microfibrils (CMFs). However, the role of MTs in oriented CMF deposition is still unclear. We have investigated the mechanism of CMF deposition with cultured tobacco protoplasts derived from taxol-treated BY-2 cells (taxol protoplasts). The BY-2 protoplasts regenerated patches of β-l,3-glucan (callose) and fibrils of β-l,4-glucan (cellulose). Taxol protoplasts possessed the same ordered MT arrays as material cells and regenerated CMFs with patterns almost coincidental with MTs. Electron microscopy revealed that, on the surface of cultured taxol protoplasts, each CMF bundle appeared to be deposited on each cortical MT. These results suggest that MTs may attach directly to the cellulose-synthesizing complexes, by some form of linkage, and regulate the movement of these complexes in higher-plant cells.
Similar content being viewed by others
Abbreviations
- CMF:
-
cellulose microfibril
- CSC:
-
cellulose-synthesizing complex
- MT:
-
microtubule
References
Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP (1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA 92: 9353–9357
Arioli T, Peng L, Betzer AS, Burn J, Wittke W, Herth W, Camilleri C, Höfte H, Plazinski J, Birch R, Cork A, Glover J, Redmond J, Williamson RE (1998) Molecular analysis of cellulose biosynthesis inArabidopsis. Science 279: 717–720
Asada T, Kuriyama R, Shibaoka H (1997) TKRP125, a kinesinrelated protein involved in the centrosome-independent organization of the cytokinetic apparatus in tobacco BY-2 cells. J Cell Sci 110: 179–189
Delmer DP, Amor Y (1995) Cellulose biosynthesis. Plant Cell 7: 987–1000
Giddings TH, Staehelin LA (1988) Spatial relationship between microtubules and plasma membrane rosettes during the deposition of primary wall microfibrils inClosterium sp. Planta 173: 22–30
— — (1991) Microtubule-mediated control of microfibril deposition: a re-examination of the hypothesis. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 85–99
Hasezawa S, Nagata T (1991) Dynamic organization of plant microtubules at the three distinct transition points during the cell cycle progression of synchronized tobacco BY-2 cells. Bot Acta 104: 206–211
— — (1993) Microtubule organizing centers in plant cells: localization of a 49 kDa protein that is immunologically cross-reactive to a 51 kDa protein from sea urchin centrosomes in synchronized tobacco BY-2 cells. Protoplasma 176: 64–74
—, Hogetsu T, Syono K (1988) Rearrangement of cortical microtubules in elongating cells derived from tobacco protoplasts: a time-course observation by immunofluorescence microscopy. J Plant Physiol 133: 46–51
— — — (1989) Changes of actin filaments and cellulose fibrils in elongating cells derived from tobacco protoplasts. J Plant Physiol 134: 115–119
Heath IB (1974) A unified hypothesis for the role of membrane bound enzyme complexes and microtubules in plant cell wall synthesis. J Theor Biol 48: 445–449
Hogetsu T, Oshima Y (1985) Immunofluorescence microscopy of microtubule arrangement inClosterium acerosum (Schrank) Ehrenberg. Planta 166: 169–175
Itano N, Hatano S (1991) F-actin bundling protein fromPhysarum polycephalum: purification and its capacity for co-bundling of actin filaments and microtubules. Cell Motil Cytoskeleton 19: 244–254
Jiang CJ, Sonobe S (1993) Identification and preliminary characterization of a 65 kDa higher-plant microtubule-associated protein. J Cell Sci 105: 891–901
Kumagai F, Hasezawa S, Takahashi Y, Nagata T (1995) The involvement of protein synthesis elongation factor-1α in the organization of microtubules on the perinuclear region during the cell cycle transition from M to G1 phase in tobacco BY-2 cells. Bot Acta 108: 467–473
Ledbetter MC, Porter KR (1963) A “microtubule” in plant cell fine structure. J Cell Biol 19: 239–250
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18: 100–127
Maeda H, Ishida N (1967) Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J Biochem 62: 276–278
Melan MA (1990) Taxol maintains organized microtubule patterns in protoplasts which lead to the resynthesis of organized cell wall microfibrils. Protoplasma 153: 169–177
Miyake T, Hasezawa S, Nagata T (1997) Role of cytoskeletal components in the migration of nuclei during the cell cycle transition from g1 phase to S phase of tobacco BY-2 cells. J Plant Physiol 150: 528–536
Nagata T, Nemoto Y, Hasezawa S (1992) Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol 132: 1–30
—, Kumagai F, Hasezawa S (1994) The origin and organization of cortical microtubules during the transition between M and g1 phases of the cell cycle as observed in highly synchronized cells of tobacco BY-2. Planta 193: 567–572
Pear JR, Kawagoe Y, Schreckengost WE, Delmer DP, Stalker DM (1996) Higher plants contain homologs of the bacterialcelA genes encoding the catalytic subunit of cellulose synthase. Proc Natl Acad Sci USA 93: 12637–12642
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–42
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hasezawa, S., Nozaki, H. Role of cortical microtubules in the orientation of cellulose microfibril deposition in higher-plant cells. Protoplasma 209, 98–104 (1999). https://doi.org/10.1007/BF01415705
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01415705