Summary
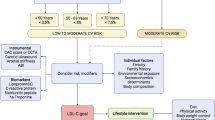
There is overwhelming evidence from prospective studies that plasma cholesterol levels are exponentially related to coronary artery disease (CAD) risk. Inversely, the beneficial effect of lowering plasma cholesterol is convincingly established from major clinical trials. A consensus has been reached in a large number of countries on the need to lower plasma lipid levels, especially LDL-cholesterol, to delay the onset, slow the progression and induce regression of atherosclerotic lesions in the coronary arteries. This remains the major indication of lipid-lowering therapy. In recent years, the emphasis has been put on target plasma lipid concentrations for dietary and drug therapy. In the process of establishing prevention strategies, however, some confusion arose: target values and criteria for assessing CAD risk and initiating therapy have differed from country to country, as well as among various groups within a country. Population strategies and high-risk case-finding strategies have clashed. Treatment algorithms have emphasized lipid levels rather than lipid transport disorders. With time, these algorithms have become more and more complex and the confused physician in practice, sometimes, has started to treat mg/dL (or mmol/L) rather than patients. This confusion has been compounded by debates on the variability of plasma lipid measurements within as well as across laboratories. In the one to one relationship that exists in the physician's office, much of this confusion can be dispelled if, after a thorough clinical evaluation, the patient's situation is taken in context, a diagnosis is made and the indicated therapy is prescribed. A good algorithm is one that focuses first on diagnosis, separates secondary from primary causes of dyslipoproteinemia, starts with diatary therapy, targets drugs to the metabolic disturbance, takes into account the psycho-social environment and the risk factor context and adjusts the treatment according to the observed response. Within this framework, specific target levels may be given due consideration. Treatment should be individualized and the key lipid transport disorders identified. Today, the physician has the advantage of prescribing drugs that have been proven valuable for the ultimate goal of therapy: prevention of atherosclerotic complications.
Similar content being viewed by others
References
Kannel WB, Neaton JD, Wentworth D, Thomas HE, Stamler J, Hulley SB, Kjelsberg MO (1986) Overall and coronary heart disease mortality rates in relation to major risk factors in 325,348 men screened for the MRFIT. Am Heart J 112: 825–836
Peto R, Yusuf S, Collins R (1985) Cholesterol-lowering trial results in their epidemiologic context (abstract). Circulation 72: 111–451
Grundy SM (1986) Cholesterol and coronary heart disease — a new era. JAMA 256: 2849–2859
Kannel WB, Castelli WP, Gordon T (1979) Cholesterol in the prediction of atherosclerotic disease-new perspectives based on the Framingham study. Ann Intern Med 90: 85–91
Lipid Research Clinics Program (1984) The Lipid Research Clinics Coronary Primary Prevention Trial Results. JAMA 251: 351–374
Frick MH, Elo O, Haapa K et al. (1987) Helsinki Heart Study: primary-prevention trial with Gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 317: 1237–1245
Manninen V, Elo O, Frick MH, et al. (1988) Helsinki Heart Study: lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart Study. JAMA 260: 641–651
Blankenhorn DH, Nessim SA, Johnson RL, Sanmarco ME, Azen SP, Cashin-Hemphill L (1987) Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA 257: 3233–3240
Ost CR, Stenson S (1967) Regression of peripheral atherosclerosis during therapy with high doses of nicotinic acid. Scand J Clin Lab Invest [Supp] 99: 241–245
Basta LL, Williams C, Kioschos JM, Spector AA (1976) Regression of atherosclerotic stenosing lesions of the renal arteries and spontaneous cure of systemic hypertension through control of hyperlipidemia. Am J Med 61: 420–422
Duffield RGM, Miller NE, Brunt JNH, Lewis B, Jamieson CW, Colchester ACF (1983) Treatment of hyperlipidaemia retards progression of symptomatic femoral atherosclerosis — a randomized controlled trial. Lancet 11: 639–642
Barndt R Jr, Blankenhorn DH, Crawford DW Brooks SH (1977) Regression and progression of early femoral atherosclerosis in treated hyperlipoproteinemic patients. Ann Intern Med 86: 139–146
Nikkilä EA, Viikinkoski P, Valle M, Frick MH (1984) Prevention of progression of coronary atherosclerosis by treatment of hyperlipidaemia: a seven year prospective angiographic study. Br Med J 289: 220–223
Brensike JF, Levy RI, Kelsey SF, et al. (1984) Effects of therapy with cholestyramine on progression of coronary arteriosclerosis: results of the NHLBI type II coronary intervention study. Circulation 69: 313–324
Buchwald H, Moore RB, Rucker RD Jr (1983) Clinical angiographic regression of atherosclerosis after partial ileal bypass. Atherosclerosis 46: 117–128
Nash DT, Gensini GG, Esente P (1983) Regression of coronary artery lesions during lipid-lowering therapy, demonstrated by scheduled serial arteriography. Int J Cardiol 3: 257–260
Watanabe Y (1980) Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit)—incidence and development of atherosclerosis and xanthoma. Atherosclerosis 36: 261–268
Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, Yoshida H, Kawai C (1987) Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci USA 84: 5928–5931
Carew TE, Schwenke DC, Steinberg DC (1987) Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoproteins degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci USA 84: 7725–7729
Walldius G, Carlson LA, Erikson U, et al. (1988) Development of femoral atherosclerosis in hypercholesterolemic patients during treatment with cholestyramine and probucol/placebo: probucol Quantitative Regression Swedish Trial (PQRST): a status report. Am J Cardiol 62: 37B-43B
Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL (1989) Beyond cholesterol. Modification of low-density lipoprotein that increase its atherogenicity. N Engl J Med 320: 915–924
Consensus Conference (1985) Lowering blood cholesterol to prevent heart disease. JAMA 253: 2080–2086
The Expert Panel (1988) Report of the National Cholesterol Education Program Expert Panel on detection, evaluation and treatment of high blood cholesterol in adults. Arch Intern Med 148: 36–69
Canadian Consensus Conference on Cholesterol (1988) Final report. The Canadian Consensus Conference on the Prevention of Heart and Vascular Disease by Altering Serum Cholesterol and Lipoprotein Risk Factors. Can Med Assoc J 139: 1–8
Study Group, European Atherosclerosis Society (1987) Strategies for the prevention of coronary heart disease: a policy statement of the European Atherosclerosis Society. Eur Heart J 8: 77–88
Warnick GR, Albers JJ, Leary ET (1980) HDL cholesterol: results of interlaboratory proficiency tests. Clin Chem 26: 169–170
Study Group, European Atherosclerosis Society (1988) The recognition and management of hyperlipidemia in adults: a policy statement of the European Atherosclerosis Society. Eur Heart J 9: 571–600
Leitch D (1989) Who should have their cholesterol measured? What experts in the United Kingdom suggest. Br Med J 298: 1615–1616
Genest JJ, Salem DN, McNamara JR, Anderson KM, Wilson PWF, Schaefer EJ (1990) Prevalence of risk factors in men with premature coronary artery disease. Application of the National Cholesterol Education Program guidelines (in press)
Wilson PFW,Christiansen JC,Anderson KM,Kannel WB (1989) Impact of national guidelines for cholesterol risk factor screening. The Framingham offspring study. JAMA 262: 41–44
Scanu AM (1989) Lipoprotein(a). Twenty five years of progress. Arteriosclerosis 9: 565–566
Davignon J, Gregg RE, Sing CF (1988) Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis 8: 1–21
Sniderman AD (1988) Apolipoprotein B and apolipoprotein AI as predictors of coronary artery disease. Can J Cardiol 4 [Suppl A]: 24A-30A
Meade TW, Brozovic M, Chakrabarti RR, et al. (1986) Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet II: 533–537
Davignon J, Xhignesse M, Roederer G (1988) Identification of the patient at risk in the physician's office and drug management of dyslipoproteinemia. Can J Cardiol 4: 36A-47A
Meade TW (1983) Factor VII and ischaemic heart disease: epidemiological evidence. Haemostasis 13: 178–185
Nestruck AC, Davignon J (1986) Risk for developing the cardiovascular risk factors: hyperlipidemia. Cardiol Clin 4: 61–70
Nikkilä EA, Aro A (1973) Family study of serum lipids and lipoproteins in coronary heart disease. Lancet I: 954–959
Soria LF, Ludwig EH, Clarke HRG, Vega GL, Grundy SM, McCarthy BJ (1989) Association between a specific apolipoprotein B mutation and familial defective apolipoprotein B-100. Proc Natl Acad Sci USA 86: 587–591
Gjone E, Norum KR, Glomset JA (1978) Familial lecithin: cholesterol acyltransferase deficiency. In: Stanbury JB, Wyngaarden JB, Fredrickson DS (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 589–603
Carlson LA, Philipson B (1979) Fish-eye disease. A new familial condition with massive corneal opacities and dyslipoproteinaemia. Lancet II: 921–923
Breckenridge CA, Little JA, Alaupovic P, Wang CS, Kiksis A, Kakis G, Lindgren F, Gardiner G (1982) Lipoprotein abnormalities associated with a familial deficiency of hepatic lipase. Atherosclerosis 45: 161–179
Breckenridge CA, Little JA, Steiner G, Chow A, Poapst M (1978) Hypertriglyceridemia associated with deficiency of apolipoprotein C-II. N Engl J Med 298: 1265–1273
Kwiterovich PO Jr, Smith HH, Connor WE, Bachorik PS, McKusick VA, Teng B, Sniderman AD (1981) Hyperapobetalipoproteinaemia in two families with xanthomas and phytosterolaemia. Lancet I: 466–469
Schaefer EJ, Heaton WH, Wetzel MG, Brewer BH Jr (1982) Plasma apolipoprotein A-I absence associated with a marked reduction of high density lipoproteins and premature coronary heart disease. Arteriosclerosis 2: 16–26
Norum RA, Lakier JB, Goldstein S, et al. (1982) Familial deficiency of apolipoprotein AI and CIII and precocious coronaryartery disease. N Engl J Med 306: 1513–1519
Ghiselli G, Schaefer EJ, Gascon P, Brewer BH Jr (1981) Type III hyperlipoproteinemia associated with apolipoprotein E deficiency. Science 214: 1239–1241
Ghiselli G, Gregg RE, Brewer B Jr (1984) Apolipoprotein E Bethesda. Isolation and partial characterization of a variant of human apolipoprotein E isolated from very low density lipoproteins. Biochim Biophys Acta 794: 333–339
Rall SC Jr, Newhouse YM, Clarke HRG, Weisgraber KH, McCarthy BJ, Mahley RW (1989) Type III hyperlipoproteinemia associated with apolipoprotein E phenotype E313. Structure and genetics of an apolipoprotein E3 variant. J Clin Invest 83: 1095–1101
Havekes L, de Witt E, Gevers-Leuen J, Klasen E, Utermann G, Weber W, Beisiegel U (1986) Apolipoprotein E3-Leiden. A new variant of apolipoprotein E associated with familial type III hyperlipoproteinemia. Hum Genet 73: 157–163
Lipid Research Clinics Program (1983) Pre-entry characteristics of participants in the Lipid Research Clinics' Coronary Primary Prevention Trial. J Chron Dis 36: 467–479
LaRosa J (1989) Review of clinical studies of bile acid sequestrants for lowering plasma lipid levels. Cardiology 76: 55–64
Carlson LA, Oro L (1973) Effect of treatment with nicotinic acid for one month on serum lipids in patients with different types of hyperlipidemia. Atherosclerosis 18: 1–9
Henwood JM, Heel RC (1988) Lovastatin. A preliminary review of its pharmacodynamic properties and therapeutic use in hyperlipidemia. Drugs 36: 429–454
Buckley MMT, Goa KL, Price AH, Brogden RN (1989) Probucol. A reappraisal of its pharmacological properties and therapeutic use in hypercholesterolemia. Drugs 37: 761–800
Franceschini G, Sirtori M, Vaccarino V, Gianfranceschi G, Rezzonico L, Chiesa G, Sirtori CR (1989) Mechanisms of HDL reduction after probucol. Changes in HDL subfractions and increased reverse cholesteryl ester transfer. Arteriosclerosis 9: 462–469
Aubry F, Lapierre Y, Noel C, Davignon J (1971) Ultracentrifugal demonstration of floating beta lipoproteins in type III hyperlipoproteinemia. Ann Intern Med 75: 231–237
Pichardo R, Boulet L, Davignon J (1977) Pharmacokinetics of clofibrate in familial hypercholesterolemia. Atherosclerosis 26: 573–582
Dujovne CA, Krehbiel P, Chernoff SB (1986) Controlled studies of the efficacy and safety of combined probucol-colestipol therapy. Am J Cardiol 57: 36H-42H
Kane JP, Malloy MJ, Tun P, Phillips NR, Freedman DD, Williams ML, Rowe JS, Havel RJ (1981) Normalization of low density lipoprotein levels in heterozygous familial hypercholesterolemia with a combined drug regimen. N Engl J Med 304: 251–258
East C, Bilheimer D, Grundy SM (1988) Combination therapy for familial combined hyperlipidemia. Ann Intern Med 109: 25–32
East C, Alivizatos PA, Grundy SM, Jones PH, Farmer JA (1988) Rhabdomyolysis in patients receiving lovastatin after cardiac transplantation. N Engl J Med 318: 47–48
Witztum JL, Simmons D, Steinberg D, et al. (1989) Intensive combination drug therapy of familial hypercholesterolemia with lovastatin, probucol, and colestipol hydrochloride. Circulation 79: 16–28
Kuo PT, Wilson AC, Kostis JB, Moreyra AB, Dodge HT (1988) Treatment of type III hyperlipoproteinemia with gemfibrozil to retard progression of coronary artery disease. Am Heart J 116: 85–90
Zelis R, Mason DT, Braunwald E, Levy RI (1970) Effect of hyperlipoproteinemias and their treatment on the peripheral circulation. J Clin Invest 49: 1007–1015
Brunzell JD, Sniderman AD, Albers JJ, Kwiterovich PO Jr (1984) Apoproteins B and A-I and coronary artery disease in humans. Arteriosclerosis 4: 79–83
Glueck CJ (1971) Effects of oxandrolone on plasma triglycerides and postheparin lipolytic activity in patients with types III, IV and V familial hyperlipoproteinemia. Metabolism 20: 691–701
Glueck CJ, Brown VW, Levy RI, Greten H, Fredrickson DS (1969) Amelioration of hypertriglyceridaemia by progestational drugs in familial type V hyperlipoproteinaemia. Lancet 1: 1290–1291
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davignon, J. Indications for lipid-lowering drugs. Eur J Clin Pharmacol 40, S3–S10 (1991). https://doi.org/10.1007/BF01409399
Issue Date:
DOI: https://doi.org/10.1007/BF01409399