Summary
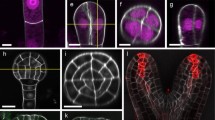
Using indirect immunofluorescence on polyethylene glycol embedded material, the organization of cortical microtubules (MTs) has been studied in explants ofNicotiana tabacum. Within 6 hours after explantation the orientation of the cortical MTs shifts from transverse to longitudinal to the long axis of the cell in all cells. This change of direction is followed by further shifts that occur only locally and predict the orientation of future cell divisions. These reorientations are independent of the formation of protrusions and buds that will develop in the explants (after 4–7 days) and they represent a stage of de-differentiation of the explants. After two days of culturing clusters of cells can be recognized, at the proximal side of the explants, with randomly oriented cortical MTs. These regions represent the origin of the protrusions from which floral buds will develop. The formation of these clusters represent the first signs of re-differentiation and formation of new polar axes in the explants. The cells thus show a very early commitment (within 2 days) as to their differentiation.
Similar content being viewed by others
Abbreviations
- BAP:
-
benzyl-amino-purine
- DMSO:
-
dimethylsulfoxid
- EGTA:
-
ethylene glycol bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- GA:
-
glutaraldehyde
- MTs:
-
microtubules
- MTOCs:
-
microtubule organizing centres
- NAA:
-
α-naphthalene acetic acid
- PEG:
-
polyethylene glycol
- PFA:
-
paraformaldehyde
- PPBs:
-
preprophase bands
References
Dustin P (1934) Microtubules. Springer, Berlin Heidelberg New York
Falconer MM, Seagull RW (1985) Xylogenesis in tissue culture: taxol effects on microtubule reorientation and lateral association in differentiating cells. Protoplasma 128: 157–166
Farrell KW, Jordan MA, Miller HP, Wilson L (1987) Phase dynamics at microtubule ends: the coexistence of microtubule length changes and treadmilling. J Cell Biol 104: 1035–1046
Green PB (1980) Organogenesis—a biophysical view. Annu Rev Plant Physiol 31: 51–82
Gunning BES, Hardham AR (1982) Microtubules. Annu Rev Plant Physiol 33: 651–698
— —,Hughes JE (1978) Evidences for initiation of microtubules in discrete regions of the cell cortex inAzolla root-tip cells, and an hypothesis on the development of cortical arrays of microtubules. Planta 143: 161–179
Hawes C, Juniper BE, Horne JC (1983) Electron microscopy of resin-free sections of plant cells. Protoplasma 115: 88–93
Kirschner M, Mitchison T (1986) Beyond self-assembly: from microtubules to morphogenesis. Cell 45: 329–342
—,Schulze E (1986) Morphogenesis and the control of microtubule dynamics in cells. J Cell Sci [Suppl] 5: 293–310
Lang JM, Eisinger WR, Green PB (1982) Effects of ethylene on the orientation of microtubules and cellulose microfibrils of Pea epicotyl cells with polylamellate cell walls. Protoplasma 110: 5–14
Lang Selker JM, Green PB (1984) Organogenesis inGraptopetalum paraguayense E. Walter: shifts in orientation of cortical microtubule arrays are associated with periclinal divisions. Planta 160: 289–297
Lloyd CW (1986) Towards a dynamic helical model for the influence of microtubules on wall patterns in plants. Int Rev Cytol 86: 1–51
Lyndon RF (1982) Changes in polarity of growth during leaf initiation in the Pea,Pisum sativum L. Ann Bot 49: 281–290
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 373–397
Quader H, Deichgraber G, Schnepf E (1986) The cytoskeleton ofCobaea seed hairs: Patterning during cell-wall differentiation. Planta 168: 1–10
—,Herth W, Ryser U, Schnepf E (1987) Cytoskeletal elements in cooton seed hair developmentin vitro: their possible regulatory role in cell well organization. Protoplasma 137: 56–62
Roberts IN, Lloyd CW, Roberts K (1985) Ethylene-induced microtubule reorientation: mediation by helical arrays. Planta 164: 439–447
Schroeder M, Wekland J, Weber K (1985) Immunofluorescence microscopy of microtubules in plant cells: stabilization by dimethylsulfoxide. Eur J Cell Biol 38: 311–318
Schulze E, Kirschner E (1986) Microtubule dynamics in interphase cells. J Cell Biol 102: 1020–1031
Steen DA, Chadwick AV (1981) Ethylene effects in Pea stem tissue. Evidence of microtubule mediation. Plant Physiol 67: 460–466
Traas JA, Braat P, Derksen JW (1984) Changes in microtubule arrays during the differentiation of cortical root cells ofRaphanus sativus. Eur J Cell Biol 34: 229–238
Tran Thanh van K (1977) Regulation of morphogenesis. In:Barz W, Reinhard E, Zenk MH (eds) Plant tissue culture and its bio-technological applications. Springer, Berlin Heidelberg New York, pp 367–385
Van den Ende G, Croes AF, Kemp A, Barendse GWM, Kroh M (1984) Development of flower buds in thin-layer tissue cultures ofNicotiana tabacum. Physiol Plant 62: 83–88
Wick SM, Duniec J (1983) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I. Preprophase band development and concomittant appearance of nuclear envelope-associated tubulin. J Cell Biol 97: 235–243
—,Seagull RW, Osborn M, Weber K, Gunning BES (1981) Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol 89: 685–690
Williams EG, Maheswaran G (1986) Somatic embryogenesis: Facors influencing coordinated behaviour of cells as an embryogenic group. Ann Bot 57: 443–462
Williams RC Jr, Caplow M, McIntosh Jr (1986) Cytoskeleton. Dynamic microtubule dynamics. Nature 324: 106–107
Wilms FHA, Sassen MMA (1987) Origin and development of floral buds in tobacco explants. New Phytol 105: 57–65
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilms, F.H.A., Derksen, J. Reorganization of cortical microtubules during cell differentiation in tobacco explants. Protoplasma 146, 127–132 (1988). https://doi.org/10.1007/BF01405921
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01405921