Summary
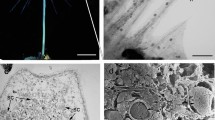
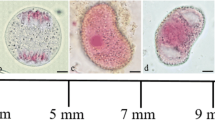
The fate of the chloroplasts and chloroplast nuclei (cp-nuclei) was followed during spermatogenesis in the fernPteris vittata L. by epifluorescence microscopy after staining with 4′-6-diamidino-2-phenylindole (DAPI) and by quantitation of chloroplast DNA (cp-DNA) by fluorimetry using a video intensified microscope photon counting system (VIMPICS). The spores were grown on solid medium that contained antheridiogen (Anptd), and formed an antheridium initial on the protonema cell. The antheridium initial divided and produced 16 spermatocytes and 3 surrounding cells. The chloroplasts in the spermatocytes decreased in volume as cell division was repeated, until finally the volume of each chloroplast was 1/15 of that of the primary chloroplasts. The DNA content of the chloroplasts was also reduced to 1/5 of the original value and when the sperm matured, the fluorescence of cp-DNA disappeared. In the 16-cell spermatocyte, the recognition of the fluorescence of chlorophyll in the chloroplasts with a green excitation filter became difficult. But, the plastids could be observed until the final stage of the sperm. From these observations, it appears that there are two steps in the metamorphosis of chloroplasts during spermatogenesis in the fern. The first step involves the decrease in the volume of chloroplasts, accompanied by reduction of the DNA content, and the second step involves the change of the physical state of chloroplasts to amyloplasts and the disappearance of the cp-DNA from the amyloplasts.
Similar content being viewed by others
References
Bell PR, Duckett JG (1976) Gametogenesis and fertilization inPteridium. J Linn Soc London Bot 73: 47–78
Boffey SA, Leech RM (1982) Chloroplast DNA and control of chloroplast division in light-grown wheat leaves. Plant Physiol 69: 1387–1391
Cavalier-Smith T (1976) Electron microscopic evidence for chloroplast fusion in zygotes ofChlamydomonas reinhardii. Nature 228: 333–335
Coleman AW (1978) Visualization of chloroplast DNA with the fluorochromes. Exp Cell Res 114: 95–100
— (1979) Use of the fluorochrome 4′-6-diamidino-2-phenylindole in genetic and developmental studies of chloroplast DNA. J Cell Biol 82: 299–305
—,Heywood P (1981) Structure of the chloroplast and its DNA in Chloromonadophycean algae. J Cell Sci 49: 401–409
—,Maguire MJ (1983) Cytological detection of the basis of uniparental inheritance of plastid DNA inChlamydomonas moewusii. Curr Genetics 7: 211–218
Duckett JG, Bell PR (1971) Studies on fertilization in archegoniate plants. I. Changes in the structure of spermatozoids ofPteridium aquilinum (L.) Kuhn during entry into the archegonium. Cytobiologie 4: 421–436
Foster AS, Gifford Jr EM (1974) Comparative morphology of vascular plants. Freeman, San Francisco
Gillham NW (1974) Genetic analysis of the chloroplast and mitochondrial genomes. Annu Rev Genet 8: 347–391
Hajduk SL (1976) Demonstration of kinetoplast DNA in dyskinetoplastic strains ofTrypanosoma equiperdum. Science 191: 858–859
James TW, Jope C (1978) Visualization by fluorescence of chloroplast DNA in higher plants by means of the DNA-specific probe 4′-6-diamidino-2-phenylindole. J Cell Biol 79: 623–630
Kuroiwa T, Suzuki T (1980) An improved method for the demonstration of thein situ chloroplast nuclei in higher plants. Cell Struct Funct 5: 195–197
—,Nishibayashi S, Kawano S, Suzuki T (1981) Visualization of DNA in various phages (T 4, χ, T 7, Φ 29) by ethidium bromide epifluorescent microscopy. Experientia 37: 969–971
—,Kawano S, Nishibayashi S (1982) Epifluorescent microscopic evidence for maternal inheritance of chloroplast DNA. Nature 298: 481–483
— (1985 a) Mechanisms of maternal inheritance of chloroplast DNA: an active digestion hypothesis. Microbiol Sci 2: 267–272
—,Enomoto S, Ishikawa I (1985) Preferential destruction of chloroplast nucleoids in zygotes in green algaeDictyosphaeria cavernosa andAcetabularia calyculus. Experientia 41: 1171–1179
—,Hori T (1986) Preferential digestion of male chloroplast and mitochondrial nuclei during gametogenesis ofBryopsis maxima. Protoplasma 133: 85–87
Lamppa GK, Elliot LV, Bendich AJ (1980) Changes in chloroplast number during pea leaf development. Planta 148: 437–443
Luttke A (1981) Heterogeneity of chloroplasts inAcetabularia mediterranea. Heterogeneous distribution and morphology of chloroplast DNA. Exp Cell Res 131: 483–488
Miyamura S, Kuroiwa T, Nagata S (1987) Disappearance of plastid and mitochondrial nucleoids during the formation of generative cells of higher plants revealed by fluorescence microscopy. Protoplasma: 141: 149–159
Myles DG, Bell PR (1975) An ultrastructural study of the spermatozoid of the fern,Marsilea vestita. J Cell Sci 17: 633–645
— (1975) Structural changes in the sperm ofMarsilea vestita before and after fertilization. In:Duckett JG, Racey PA (eds) The biology of the male gamete. Academic Press, London, pp 129–134
—,Hepler PK (1977) Spermiogenesis in the fernMarsilea: microtubules, nuclear shaping, and cytomorphogenesis. J Cell Sci 23: 57–83
— (1978) The fine structure of fertilization in the fernMarsilea vestita. J Cell Sci 30: 265–281
Possingham JV, Chang N, Robertson MA, Cain PA (1983) Studies of the distribution of DNA within spinach plastids. Biol Cell 47: 205–212
—,Lawrence ME (1983) Control of plastid division. Int Rev Cytol 84: 1–56
Sager R, Ramanis Z (1973) The mechanism of maternal inheritance inChlamydomonas: biochemical and genetic studies. Theor Appl Genetics 43: 101–108
Scott NS, Possingham JV (1980) Chloroplast DNA in expanding spinach leaves. J Exp Bot 31: 1081–1092
— — (1982) In:Nagley P, Lumane AW, Peacock JW, Paleman JA (eds) Manipulation and expression of genes in eukaryotes. Academic Press, New York, pp 255–256
Sears BB (1980) Review: elimination of plastids during spermatogenesis and fertilization in the plant kingdom. Plasmid 4: 233–255
Williamson DH, Fennell DJ (1975) The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. In:Prescott DM (ed) Methods in cell biology, vol 112. Academic Press, New York, pp 335–351
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuroiwa, H., Sugai, M. & Kuroiwa, T. Behavior of chloroplasts and chloroplast nuclei during spermatogenesis in the fern,Pteris vittata L.. Protoplasma 146, 89–100 (1988). https://doi.org/10.1007/BF01405917
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01405917