Summary
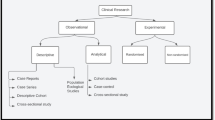
The growing difference with regard to academic and professional accountability between neurosurgeons and neuroscience is alarming. Controlled clinical trials continue to be the most scientifically valid method of evaluating standard treatments against the risks and benefits of medical innovations. Clinical research becomes more rewarding as neurosurgeons gain familiarity with the particulars of the structure and relevance of expertly designed clinical cooperative trials. The basic principles and the significance of biometrical essentials (research hypothesis, comparability, randomized and nonrandomized trials, phases of studies and planning steps) are briefly discussed. A guide to the pertinent literature is provided.
Similar content being viewed by others
References
Anonymous (1987) Committee on Health Care Issues, American Neurological Association: Does carotid endarterectomy decrease stroke and death in patients with transient ischemic attacks? Ann Neurol 22: 72–76
Anonymous (1985) The EC/IC Bypass Study Group: The international cooperative study of extracranial/intracranial arterial anastomosis (EC/IC Bypass Study): Methodology and entry characteristics. Stroke 16: 397–406
Anonymous (1985) The EC/IC Bypass Study Group: Failure of extracranial/intracranial arterial bypass to reduce the risk of ischemic stroke: Results of the international randomized trial. N Engl J Med 313: 1191–1200
Elston RC, Johnson WD (1987) Essentials of biostatistics. F.A. Davis Company, Philadelphia, pp 246–251
Enthoven AC (1985) The changing economic context of medical decision making. In: Clinical neurosurgery. Proceedings of the congress of neurological surgeons, Honolulu, Hawaii, 1985. Williams & Wilkins, Baltimore, London, Los Angeless, Sydney, pp 81–91
Goldring S, Zervas N, Langfitt T (1987) The extracranial/intracranial bypass study: A report of the committee appointed by the American Association of Neurological Surgeons to examine the study. N Engl J Med 316: 817–820
Haines SJ (1981) Six statistical suggestions for surgeons. Neurosurgery 9: 414–418
Hulley SB, Cummings SR (1988) Designing clinical research. An epidemiologic approach. Williams & Wilkins, Baltimore, Hongkong, London, Sydney
Jacobs SK, Wilson DJ, Kornblith BL,et al (1986) In vitro killing of human glioblastoma by interleukin-2-activated autologous lymphocytes. J Neurosurg 64: 114–117
McDowell FH (1988) Neurology, Neurosurgery, Controlled trials and academic accountability. Stroke 19: 1463–1465
Plum F (1985) Extracranial-intracranial arterial by-pass and cerebral vascular disease. N Engl J Med 313: 1221–1223
Sacks H, Chalmers TC, Smith H (1982) Randomized versus historical controls for clinical trials. Am J Med 72: 233–240
Suggested Literature
Biefang S, Köpke W, Schreiber MA (1979) Manual für die Planung und Durchführung von Therapiestudien. Medizinische Informatik und Statistik, Bd 13. Springer, Berlin, Heidelberg, New York, Tokyo
Friedmann LM, Furberg CD, DeMets DL (1985) Fundamentals of clinical trials. 2nd ed. PSG Publishing Comp, Littleton, Ma
Hulley SB, Cummings SR (1988) Designing clinical research. An epidemiologic approach. Williams & Wilkins, Baltimore, Honkong, London, Sydney
Kelsey JF, Thompson WD, Evans AS (1986) Methods in observational epidemiology. Oxford University Press, New York
Kleinbaum DG, Kupper LL, Morgenstern H (1982) Epidemiologic research: Principles and quantitative methods. Lifetime Learning Publications, Belmont, Cal
Meinert C (1986) Clinical trials. Oxford University Press, New York
Pocock SJ (1983) Clinical trials: A practical approach. John Wiley and Sons, Chichester
Schlesselman JJ (1982) Case-control studies: Design, conduct, analysis. Oxford University Press, New York 1982
Silvermann WA (1985) Human experimentation: A guided step into the unknown. Oxford University Press, Oxford, New York
Spilker B (1984) Guide to clinical studies and developing protocols. Raven Press, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ostertag, C.B., Werdier, D., Barcia, J. et al. The clinical cooperative trial guide to a critical approach. Acta neurochir 110, 1–5 (1991). https://doi.org/10.1007/BF01402040
Issue Date:
DOI: https://doi.org/10.1007/BF01402040