Summary
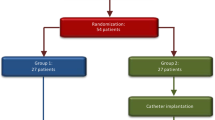
Among a series of 224 patients with aneurysmal subarachnoid haemorrhage (SAH) admitted over a period of three years, 52 patients were prospectively treated with intrathecal tissue plasminogen activator (rTPA). All of these patients were admitted and operated on within 72 h after SAH. SAH was confirmed by CT scan and the volume of blood accumulated in the basal cisterns was graded according to Fisher's scale. All patients had a SAH according to Fisher's grade III, as a prerequisite for inclusion into the study. In 21 patients additional intraventricular bleeding was detectable on CT scan. The diagnosis of a single intracerebral aneurysm as the bleeding source was established by pan-angiography, which also excluded additional cerebro-vascular malformations.
The control group consisted of 68 patients, which were also treated within 72 h after SAH. Age and sex distribution as well as the clinical patterns were comparable to the rTPA group.
In all patients the aneurysm was clipped using standard microsurgical techniques. After the aneurysm had been excluded from the parent vessel, 10 mg of rTPA, dissolved in 10 ml of its solution fluid, were slowly instilled into the basal cisterns in the treatment group. In patients with additional severe intraventricular bleeding, 5–10 mg of rTPA were injected into the ventricles via an intraventricular catheter at the end of the operation. Apart from the intrathecal application of the thrombolytic substance, the surgical protocol was identical in the patients of the control group.
During the postoperative period, the patients in both groups were examined neurologically and by transcranial Doppler on a daily basis. CT scans were performed on days 1, 2, 5, 10 postoperatively and immediately prior to discharge. Final clinical grading for this study was performed three months after surgery and the patients were graded according to the Glasgow Outcome Scale. The occurrence of clinical signs of delayed ischaemic deficits (DID), attributable to the occurrence of cerebral vasospasm, was the only defined endpoint of the study. Radical blood clot removal, verfied by serial CT scans was achieved in all patients treated using the intrathecal thrombolytic agent.
Overall results in the rTPA group at three months postsurgery were as follows: 39 patients (75%) were in grade I, 7 in grade II (13.5%), and 6 patients (11.5%) were in grade III GOS. Delayed ischaemic deficits, attributable to the occurrence of vasospasm were apparent in 4 patients (8%), in whom clinical symptoms were moderate in two patients and severe in another two. Three patients responded well to moderate hypertensive-hypervolaemic treatment resulting in an increase of their systolic arterial pressure up to 160 mm Hg. In none of these three patients cerebral infarction and/or permanent neurological deficits developed. In one patient with spasmogenic infarction of the middle cerebral artery territory in complete hemiparesis persisted. The overall results in the control group were as follows: 44 patients (64%) were in grade I GOS postoperatively, 6 in grade II (9%), 14 in grade III (21%), 1 in grade IV (1.5%), and three patients (4%) had died. DID attributable to the development of vasospasm developed in 16 patients (23.5%). DID were transient in 9 patients (13%) resolving completely after induction of hypertensive and hypervolaemic therapy. In four patients (6%) neurological deficits persisted despite vigorous treatment, and 3 patients (4%) died from spasmogenic cerebral infarction.
From the results of this first prospective study of a single bolus injection of rTPA in patients with aneurysm rupture, it is concluded, that intrathecal thrombolysis is an effective and safe method for removal of intracisternal blood accumulations after SAH resulting in a significant reduction of symptomatic vasospasm and DID. With regard to the radicality of blood clot removal achievable by the use of rTPA it is furthermore concluded, that conversion of a SAH according to Fisher grade III into a SAH of Fisher grade II is sufficient for significant reduction of the incidence of posthaemorrhagic DID, avoiding the necessity of complete pharmacological blood clot evacuation and the use of higher concentrations of rTPA or continuous irrigation of the subarachnoid space.
Similar content being viewed by others
References
Alksne JF, Branson PJ, Bailey M (1988) Modification of experimental post-subarachnoid hemorrhage vasculopathy with intracisternal plasmin. Neurosurgery 23: 335–337
Ecker A, Riemenschneider PA (1951) Arteriographic demonstration of spasm of intracranial arteries with special reference to saccular arterial aneurysms. J Neurosurg 8: 660–667
Eisert WG, Callisen H, Müller TH (1987) Multiple bolus injection of rTPA lyse clots in the rabbit jugular vein model as efficient as continuous infusion. J Cell Biochem 11 [Suppl]: 184
Findlay JM, Weir BKA, Steinke D, Tanabe T, Gordon P, Grace P (1988) Effect of intrathecal thrombolytic therapy on subarachnoid clot and chronic vasospasm in a primate model of SAH. J Neurosurg 69: 723–735
Findlay JM, Weir BKA, Kanamuru K, Grace M, Gordon P, Baughman R, Howarth A (1989) Intrathecal fibrinolytic therapy after subaracbnoid hemorrhage: dosage study in a primate model and review of the literature. Can J Neurol Sci 16: 28–40
Findlay JM, Weir BKA, Kassell NF, Disney LB, Grace MGA (1991) Intracisternal recombinant tissue plasminogen activator after aneurysmal subarachnoid hemorrhage. J Neurosurg 75: 181–188
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6: 1–9
Fraser J, Johnson S, Ray M, Robertson JT (1980) Prediction of cerebral vasospasm with subarachnoid hemorrhage due to ruptured intracranial aneurysm by computerized tomography. Neurosurgery 6: 686–687
Fujii S, Fujitsu K (1988) Experimental vasospasm in cultured arterial smooth-muscle cells. Part 1: contractile and ultrastructural changes caused by oxyhemoglobin. J Neurosurg 69: 92–97
Handa Y, Weir BKA, Nosko M, Mosewich R, Tsuji T, Grace M (1987) The effect of timing of clot removal on chronic vasospasm in a primate model. J Neurosurg 67: 558–564
Hunt WE, Hess RM (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysm. J Neurosurg 28: 14–19
Hunt WE, Kassell N, Pertuiset B, Sano K, Teasdale G, de Villier J, Drake CG (1988) Report of the World Federation of Neurological Surgeons Committee on a universal subarachnoid haemorrhage grading scale. J Neurosurg 68: 985–986
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet i: 480–484
Johnson R, Potter JM, Reid RG (1958) Arterial spasm in subarachnoid hemorrhage: mechanical considerations. J Neurol Neurosurg Psychiatry 21: 68
Kaufman HH, Schochet S, Koss W, Herschberger RN, Bernstein D (1986) Efficacy and safety of tissue plasminogen activator. Neurosurgery 20: 403–407
Kistler JP, Crowell RM, Davis KR, Heros R, Ojeman RG, Zervas T, Fisher CM (1983) The relation of cerebral vasospasm to the extent and location of subarachnoid blood visualized by CT scan. A prospective study. Neurology 33: 424–436
Mac Donald RL, Weir BKA, Saito K, Kanamura K, Findlay JM, Grace M, Runzer T, Mielke B (1991) Etiology of cerebral vasospasm in primates. J Neurosurg 75: 415–424
Mizukami M, Kawase T, Usami T, Tazawa T (1981) Prevention of vasospasm by early operation with removal of subarachnoid blood. Neurosurgery 10: 301–307
Nosko M, Weir BKA, Lunt A, Grace M, Allen P, Miehlke B (1987) Effect of clot removal at 24 hours on chronic vasospasm after SAH in a primate model. J Neurosurg 66: 416–422
Öhman J, Servo A, Heiskanen O (1991) Effect of intrathecal fibrinolytic therapy on clot lysis and vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 75: 197–201
Ohta H, Ito Z, Yasui N (1982) Extensive evacuation of subarachnoid clot for prevention of vasospasm — effective or not? Acta Neurochir (Wien) 67: 394–398
Osaka K (1977) Prolonged vasospasm produced by breakdown products of erythrocytes. J Neurosurg 47: 403–411
Pennica D, Holmes WE, Kohr WJ (1983) Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature 301: 214–221
Seifert V, Stolke D, Kaever V, Dietz H (1987) Arachidonic acid metabolism after aneurysm rupture — evaluation of 6-Keto-PGF 1 alpha and TXB 2 in patients with subarachnoid hemorrhage. Surg Neurol 27: 243–252
Seifert V, Stolke D, Kunz U, Resch K (1988) Influence of blood volume on cerebrospinal fluid levels of arachidonic acid metabolites after subarachnoid hemorrhage: experimental study on the pathogenesis of cerebral vasospasm. Neurosurgery 23: 313–321
Seifert V, Eisert WG, Stolke D, Goetz Ch (1989) Efficacy of single intracisternal bolus injection of recombinant tissue plasminogen activator to prevent delayed cerebral vasospasm after experimental subarachnoid hemorrhage. Neurosurgery 25: 590–598
Seifert V, Stolke D (1990) Injection of tissue plasminogen activator. Neurosurgery 26: 549–550
Shiobara R, Kawase T, Toya S, Ebato K, Miyahara Y (1985) “Scavenger surgery” for subarachnoid hemorrhage. II. Continuous ventriculocisternal perfusion using artificial cerebrospinal fluid with urokinase. In: Auer LM (ed) Timing of aneurysm surgery. De Gruyter, Berlin, pp 365–372
Stolke D, Seifert V (1991) Single intracisternal bolus of recombinant tissue plasminogen activator in patients with aneurysmal subarachnoid hemorrhage: preliminary assessment of efficacy and safety in an open clinical study. Neurosurgery 30: 877–881
White RP, Robertson JT (1987) Pharmacodynamic evaluation of human cerebral arteries in the genesis of vasospasm. Neurosurgery 21: 523–531
Yoshida Y, Ueki S, Takahashi A (1985) Intrathecal irrigation with urokinase in ruptured cerebral aneurysm cases. Basic studies and clinical application. Neurol Med Chir 24: 987–997
Zabramski JM, Spetzler RF, Bonstelle C (1986) Chronic cerebral vasospasm: effect of volume and timing of hemorrhage in a canine model. Neurosurgery 18: 1–6
Zabramski JM, Spetzler RF, Lee KSt, Papadopoulos StM, Bovill E, Zimmermann RS, Bederson JA (1991) Phase I trial of tissue plasminogen activator for prevention of vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 75: 189–196
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seifert, V., Stolke, D., Zimmermann, M. et al. Prevention of delayed ischaemic deficits after aneurysmal subarachnoid haemorrhage by intrathecal bolus injection of tissue plasminogen activator (rTPA). Acta neurochir 128, 137–143 (1994). https://doi.org/10.1007/BF01400664
Issue Date:
DOI: https://doi.org/10.1007/BF01400664