Summary
A convenient preparation of the 14-membered macrocyclic diamide 5,7-dioxo-1,4,8,11-tetraazacyclotetradecane (LH2) is described. The pK +NH values of the ligand are pK1 = 5.76 and pK2 = 9.63 at 25° and I = 0.1 mol dm−3 (KNO3). With metal ions able to ionise amide hydrogens, the ligand acts as a planar quadridentate, L2−. Thus copper(II) and nickel(II) give the neutral complexes ML, and conductivity measurements confirm that they are nonelectrolytes in aqueous solution. Both the nickel(II) and copper(II) complexes are acid labile unlike the analogues of 1,4,8,11-tetraazacyclotetradecane (cyclam).
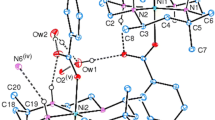
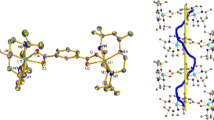
The cobalt(III) complex [CoL(NH3)2]Cl has been characterised and1H n.m.r. measurements established the N-meso stereochemistry at the chiral nitrogen centres.
Similar content being viewed by others
References
I. Tabushi, Y. Taniguchi and H. Kato,Tetrahedron Lett., 1049 (1977).
H. Kato, Doctoral Thesis, Department of Pharmaceutical Sciences, Kyushu University, 1977.
M. Kodama and E. Kimura,J. Chem. Soc. Dalton Trans., 325 (1979).
H. A. O. Hill and K. A. Raspir,J. Chem. Soc. A, 3036 (1968).
F. P. Bossu, K. L. Chelloppa and D. W. Margerum,J. Am. Chem. Soc., 99, 2195 (1977).
A. G. Lappin, C. K. Murray and D. W. Margerum,Inorg. Chem., 17, 1630 (1978).
E. Zeigerson, G. Ginzburg, N. Schwartz, Z. Luz and D. Meyerstein,J. Chem. Soc. Chem. Comm., 241 (1979).
A. A. Hinz and D. W. Margerum,Inorg. Chem., 13, 2941 (1974).
See for example, S. P. Datta and B. R. Rabin,Trans. Faraday Soc., 52, 1123 (1956).
R. B. Martin, M. Chamberlin and J. T. Edsall,J. Am. Chem. Soc., 82, 495 (1960).
R. D. Gillard, E. D. McKenzie, R. Mason and G. B. Robertson,Coordination Chem. Rev., 1, 263 (1966).
A. Albert and E. P. Serjeant,Ionisation Constants of Acids and Bases, Methuen London, 1962.
M. Kato,Z. phys. Chem. Frankfurt, 23, 391 (1960).
K. Nakanishi,Infrared Absorption Spectroscopy, Holden-Day, San Francisco, 1962.
U. Sakaguchi, K. Morito and H. Yoneda, Chem. Lett., 19 (1979).
D. A. Buckingham, L. Durham and A. M. Sargeson,Austr. J. Chem., 20, 257 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hay, R.W., Norman, P.R. The coordination chemistry of macrocyclic diamides. Transition metal complexes of 5,7-dioxo-1,4,8,11-tetraazacyclotetradecane. Transition Met Chem 5, 232–235 (1980). https://doi.org/10.1007/BF01396922
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01396922