Abstract
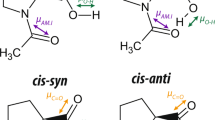
The effects of hydrogen bonding on the geometry of the carboxyl group have been studied systematically based on accurate X-ray crystallographic data. In general, the C-O(H) bond length increases with increasing O ⋯ O hydrogen-bond length, while the C=O bond length decreases. These variations become less pronounced for longer O ⋯ O distances. The O=C-O(H) and C-C-O(H) angles decrease with increasing O ⋯ O separation, while the C-C=O angle increases. The sum of the three angles remains close to 360 °, testifying to the planarity of the carboxyl group. These correlations are not observed to hold for cyclic hydrogen-bonded dimers, indicating that a continuous variation in the degree of disorder of the protons may be present, ranging from the ordered case with easily distinguishable C=O and C-O(H), to the 50%-50% disordered case, where the two C-O distances become equal.
Similar content being viewed by others
References
Currie, M., Speakman, J. C., and Curry, N. A. (1967)J. Chem. Soc. A, 1862.
Dieterich, D. A., Paul, I. C., and Curtin, D. Y. (1974)J. Am. Chem. Soc. 96, 6372.
Ichikawa, M. (1978)Acta Cryst. B 34, 2074.
Ichikawa, M. (1979)Acta Cryst. B 35, 1300.
Leiserowitz, L. (1976)Acta Cryst. B 32, 775.
Nahringbauer, I. (1970)Acta Univ. Ups. (Abstracts of Uppsala Dissertations from the Faculty of Science), No. 157.
Olovsson, I., and Jönsson, P. -G. (1967)The Hydrogen Bond, Vol. II, P. Schuster, G. Zundel, and C. Sandorfy, eds. (North-Holland, Amsterdam), Chap. 8.
Osaki, K. (1975)J. Cryst. Soc. Jpn 17, 10.
Pringle, G. E., and Broadbent, T. A. (1965)Acta Cryst. 19, 426.
Speakman, J. C. (1972)Struct. Bond. 12, 141.
Takusagawa, F., Hirotsu, K., and Shimada, A. (1973)Bull. Chem. Soc. Jpn. 46, 2020.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ichikawa, M. The effect of hydrogen bonding on the bond lengths and angles in the carboxyl group. Journal of Crystal and Molecular Structure 9, 87–105 (1979). https://doi.org/10.1007/BF01387561
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01387561