Abstract
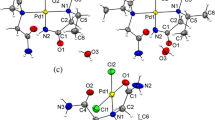
The crystal and molecular structure oftrans-dichlorobis(oxazole)palladium-(II), Pd(C3H3ON)2Cl2, has been determined by single-crystal X-ray diffraction techniques using counter methods and has been refined by full-matrix least-squares procedures to a finalR index of 0.022. The complex crystallizes in the triclinic space groupP¯1 with unit cell dimensions ofa = 6.957(1),b = 7.506(1),c = 5.538(1) Å, α = 109.22(1), β = 91.37(1), and γ = 115.09(1) °, withZ = 1. The palladium(II) ion, located at an inversion center, is coordinated in a regular square-planar manner to two chloride ions at 2.293(1) Å and, at 2.016(2) Å, to the nitrogen atoms of two oxazole ligands. The planar oxazole rings are tilted 33 ° with respect to the PdN2Cl2 plane. Further verification of the presence of oxazole in the complex was obtained by high-resolution mass spectrometry. Crystals of Pd(C3H3ON)2Cl2 resulted irreproducibly from attempts to prepare single crystals of a previously reported dimeric palladium(II) disulfide complex by treating Pd(CH3CN)2Cl2 with a slight excess of diphenyl disulfide in benzene in the atmosphere. The source of the oxazole is not understood.
Similar content being viewed by others
References
Albano, V., Bellon, P. L., Pompa, F., and Scatturin, V. (1963)Ric Sci. 33, 1143.
Ambats, I., and Marsh, R. E. (1965)Acta Cryst. 19, 942.
Battaglia, L. P., Bonamartini Corradi, A., Grasselli Palmieri, C., Nardelli, M., and Vidoni Tani, M. E. (1973)Acta Cryst. B 29, 762.
Boschi, T., Crociani, B., Toniolo, L., and Belluco, U. (1970)Inorg. Chem. 9, 532.
Busing, W. R., and Levy, H. A. (1957)Acta Cryst. 10, 180.
Chieh, P.C. (1972)J. Chem. Soc. Dalton Trans., 1643.
Freeman, H. C., and Snow, M. R. (1965)Acta Cryst. 18, 843.
Gantzel, P. K., Sparks, R. A., and Trueblood, K. N. (1964) UCLALS4, American Crystaliographic Association Program Library (old) No. 317, modified by T. Ottersen and K. Seff (1976).
International Tables for X-Ray Crystallography (1974a) Vol. IV (Kynoch Press, Birmingham, England), p. 55.
International Tables for X-Ray Crystallography (1974b) Vol. IV (Kynoch Press, Birmingham, England), p. 72.
International Tables for X-Ray Crystallography (1974c) Vol. IV (Kynoch Press, Birmingham, England), p. 149.
Ito, T., Marumo, F., and Saito, Y. (1971)Acta Cryst. B 27, 1062.
Johnson, C. K. (1965)Ortep. (Report ORNL-3794, Oak Ridge National Laboratory, Oak Ridge, Tennessee).
Khare, G. P., Little, R. G., Veal, J. T., and Doedens, R. J. (1975)Inorg. Chem. 14, 2475.
Kolthoff, I. M., and Striks, W. (1951)J. Am. Chem. Soc. 73, 1728.
Little, R. G., and Doedens, R. J. (1973)Inorg. Chem. 12, 537.
Martin, L. L., and Jacobson, R. A. (1971)Inorg. Chem. 10, 1795.
Mokuolu, J. A. A., Payne, D. S., and Speakman, J. C. (1973)J. Chem. Soc. Dalton Trans., 1443.
Ottersen, T. (1974) Computer ProgramAbsco, Department of Pharmacy, University of Oslo.
Ottersen, T. (1976) Computer ProgramLp-76, Department of Chemistry, University of Hawaii.
Pauling, L. (1960)The Nature of the Chemical Bond, 3rd edn. (Cornell University Press, Ithaca, New York), pp. 253–254.
Petersen, S. W., and Levy, H. A. (1957)Acta Cryst. 10, 70.
Prout, C. K., and Wheeler, A. G. (1966)J. Chem. Soc. A, 1286.
Sinn, E., Flynn, Jr., C. M., and Martin, R. B. (1977)Inorg. Chem. 16, 2403.
Wayland, B. B., and Schramm, R. F. (1969)Inorg. Chem. 8, 971.
Zanella, R., Ros, R., and Graziani, M. (1973)Inorg. Chem. 12, 2736.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Binamira-Soriaga, E., Lundeen, M. & Seff, K. Crystal and molecular structure oftrans-dichlorobis(oxazole)palladium(II). Journal of Crystal and Molecular Structure 9, 67–75 (1979). https://doi.org/10.1007/BF01387559
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01387559