Summary
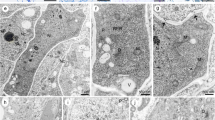
Changes in the lysosome structures were examined by electron microscopy during the formation of zoospores inTrebouxia potteri. Lysosomes in vegetative cells were homogeneously filled with electron-dense material. At the beginning of zoospore formation, lysosomes invaginated or evaginated to take up mitochondria, ER, or cytoplasmic ground plasma. The ingested organelles became disorganized within the lysosomes. During this disruption of these organelles, the lysosomal contents became heterogeneous, suggesting a decrease in the amount of enzymes within the lysosomes. Golgi bodies and ER seemed to be involved with the disruption of the organelles, probably supplying some substances necessary for the functioning of the lysosomes. Amount of electron-dense materials decreased and, finally, only one to three small spherical aggregates remained in the lysosomes. Then the lysosomes appeared to shrink via loss of watery substances or cutting off of electron-transparent regions. After these changes in lysosome structure, nuclei started to divide successively for formation of the zoospores. The possibility is proposed that the drastic cytoplasmic changes operated by lysosomes trigger the following morphogenetic events in the formation of zoospores.
Similar content being viewed by others
Abbreviations
- ER:
-
endoplasmic reticulum
- TGN:
-
trans Golgi network
References
Bainton D (1981) The discovery of lysosomes. J Cell Biol 91: 66s-76s
Berjak P (1972) Lysosomal compartmentation: ultrastructural aspects of the origin, development and function of vacuoles inLepidium sativum. Ann Bot 36: 73–81
Chida Y, Ueda K (1991) Division of chloroplasts in a green alga,Trebouxia potteri. Ann Bot 67: 435–442
Fineran BA (1972) Ultrastructure of vacuolar inclusions in root tips. Protoplasma 72: 1–18
Gray RH, Brabec RK, Cox SG, Foster PW, Bernstein IA (1974) The ultrastructural localization of glucose-6-phosphatase and possible origin of concentric membranes of hepatic autophagic vacuoles. J Cell Biol 63: 120 a
Hara-Nishimura I, Hayashi M, Nishimura M, Akazawa T (1987) Biogenesis of protein bodies by budding from vacuoles in developing pumpkin cotyledons. Protoplasma 136: 49–55
Ichimura T (1971) Sexual cell division and conjugation-papilla formation in sexual reproduction ofClosterium strigosum. In: Nishizawa K et al (eds) Proceedings of the 7th International Seaweed Symposium. University of Tokyo Press, Tokyo, pp 208–214
Leedale GF, Buetow DE (1976) Observations on cytolysome formation and other cytological phenomena in carbon-starvedEuglena gracilis. J Microsc Biol Cell 25: 149–154
Locke M, McMahon JT (1971) The origin and fate of microbodies in the fat body of an insect. J Cell Biol 48: 61–78
Marty F (1970) Rôle du système membranaire vacuolarie dans la differéntiation des laticifères d'Euphorbia characians L. CR Acad Sci D 271: 2301–2304
— (1978) Cytochemical studies on GERL, provacuoles, and vacuoles in root meristematic cells ofEuphorbia. Proc Natl Acad Sci USA 75: 852–856
Marzella L, Ahlberg J, Glaumann H (1980) In vitro uptake of particles by lysosomes. Exp Cell Res 129: 460–466
Matile P (1975) The lytic compartment of plant cells. Springer, Wien New York [Alfert M et al (eds) Cell biology monographs, vol 1]
— (1978) Biochemistry and function of vacuoles. Annu Rev Plant Physiol 29: 193–213
Mayahara H, Chang JP (1978) Electron microscopic study of acid phosphatase activity in cultured human cystic fibrosis fibroblasts. Acta Histochem Cytochem 11: 449–459
Mesquita JF (1972) Ultrastructure de formations comparables aux vacuoles autophagiques dans les cellules des racines de l'Allium cepa L. et duLupinus albus L. Cytologia 37: 95–110
Nishimura M, Beevers H (1978) Hydrolases in vacuoles from castor bean endosperm. Plant Physiol 62: 44–48
Noguchi T (1976) Phosphatase activities and osmium reduction in cell organelles ofMicrasterias americana. Protoplasma 87: 163–178
— (1990) Consumption of lipid granules and formation of vacuoles in the pollen tube ofTradescantia reflexa. Protoplasma 156: 19–28
Novikoff AB, Shin WY (1978) Endoplasmic reticulum and autophagy in rat hepatocytes. Proc Natl Acad Sci USA 75: 5039–5042
Peoples MB, Beilharz VC, Waters SP, Simpson RJ, Dalling MJ (1980) Nitrogen redistribution during grain growth in wheat (Triticum sativum L.). II. Chloroplast senescence and the degradation of ribulose-1,5-bisphosphate carboxylase. Planta 149: 241–251
Reunanen H, Hirsimäki P (1983) Studies on vinblastine-induced autophagocytosis in mouse liver. IV. Origin of membranes. Histochemistry 79: 59–67
Saito T, Ogawa K (1974) Lysosomal changes in rat hepatic parenchymal cells after glucagon administration. Acta Histochem Cytochem 7: 1–8
Sakai M, Ogawa K (1984) Relationship between lysosomal wrapping mechanism (LWM) and cytoskeletal elements during autophagolysosome formation. Acta Histochem Cytochem 17: 1–14
Smith RE, Farquhar MG (1966) Lysosome function in the regulation of the secretory process in cells of the anterior pituitary gland. J Cell Biol 31: 319–347
Tan K, Ueda K (1978) Rapid degeneration of the protoplasm in artificially induced small cells ofMicrasterias crux melitensis. Protoplasma 97: 61–70
Vaughn KC, Duke SO (1981) Evaginations from the plastid envelope: a method for transfer of substances from plastid to vacuole. Cytobios 32: 89–95
Villiers TA (1971 a) Lysosomal activities of the vacuole in damaged and recovering plant cells. Nature New Biol 233: 57–58
— (1971 b) Cytology studies in dormancy. II. Pathological ageing changes during prolonged dormancy and recovery upon dormancy release. New Phytol 71: 145–152
von Figura K, Hasilik A (1986) Lysosomal enzymes and their receptors. Annu Rev Biochem 55: 167–193
Vorbrodt A, Gruca S, Gruca-Krzyzowska S (1971) Cytochemical studies on the participation of endoplasmic reticulum in the formation of lysosomes (dense bodies) in rat hepatocytes. J Microscopie 12: 73–82
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chida, Y., Ueda, K. Changes in the lysosome structure during the formation of zoospores inTrebouxia potteri . Protoplasma 171, 19–27 (1992). https://doi.org/10.1007/BF01379276
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01379276