Abstract
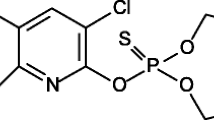
The sorption of 4-nitrophenol (4-NP) in enzymatically isolated cuticles ofLycopersicon esculentum fruits andFicus elastica leaves was studied as a function of temperature and solute concentration. Plots of the concentrations of 4-NP sorbed in the cuticle versus the equilibrium concentrations in the aqueous phase gave linear isotherms at low concentrations that tended to approach plateaus at higher sorbate concentrations (≥ 10 mmol·kg-1). At low concentrations of sorbed 4-NP, cuticles have sorptive properties similar to those of organic solvents which are able to form intermolecular hydrogen bonds, while at higher concentrations their solid nature becomes apparent. During sorption of 4-NP the cutin matrix swells and new sorption sites are successively formed. The partition coefficients of 4-NP in the system cuticle/buffer are functions of temperature and concentration. At high sorbate concentrations (approx. 1 mol·kg-1) they approach a value of 1. Different sorptive properties were observed for the cutin regions normally encrusted with soluble cuticular lipids (SCL) and those without SCL. Increasing temperature augmented the number of sorption sites in the cutin ofLycopersicon while no effect was observed withFicus. The changes of partial molar free energy (ΔG o tr), enthalpy (ΔH o tr), and entropy (ΔS o tr) for the phase transfer of 4-NP also depended on sorbate concentration: ΔH o tr and ΔS o tr were negative and steeply decreased at high sorbate concentrations. This is due to solute-solute interactions replacing solute-cutin interactions at high concentrations resulting in solid precipitates of solute within the cutin matrix. This formation of ordered solid domaines starting from a small number of nonelectrolyte molecules interacting with the cutin is proposed as a model for the intracuticular deposition of SCL.
Similar content being viewed by others
Abbreviations
- CM:
-
cuticular membrane
- MX:
-
polymer matrix membrane
- 4-NP:
-
4-nitrophenol
- SCL:
-
soluble cuticular lipids
References
Adamson, A.W. (1982) Physical chemistry of surfaces. 4th edn., Wiley, New York
Baker, E.A. (1982) Chemistry and morphology of plant epicuticular waxes. In: The plant cuticle, pp. 139–165, Cutler, D.F., Alvin, K.L., Price, C.E., eds. Academic Press London New York
Chambers, T.C., Ritchie, I.M., Booth, M.A. (1976) Chemical models for plant wax morphogenesis. New Phytol.77, 43–49
Deas, A.H.B., Holloway, P.J. (1977) The intermolecular structure of some plant cutins. In: Lipids and lipid polymers in higher plants, pp. 293–299, Tevini, M., Lichtenthaler, H.K. eds, Springer, Berlin Heidelberg New York
Diamond, J.M., Katz, Y. (1974) Interpretation of nonelectrolyte partition coefficients between dimyristoyl lecithin and water. J. Membr. Biol.17, 121–154
Eckl, K., Gruler, H. (1980) Phase transitions in plant cuticles. Planta150, 102–113
Fox, R.C. (1958) The relationship of wax crystal structure and the water vapor transmission rate of wax films. TAPPI41, 283–289
Franz, H.-P., Bartusch, W., Heiss, R. (1972) Untersuchungen über die Wasserdampfdurchlässigkeit paraffinbeschichteter Papiere. Fette, Seifen, Anstrichmitt.74, 469–475
Giles, C.H., MacEwan, T.H., Nakhwa, S.N., Smith, D. (1960) Studies in adsorption XI. J. Chem. Soc.1960, 3973–3993
Giles, C.H., Nakhwa, S.N. (1962) Studies on adsorption XVI. J. Appl. Chem.12, 266–273
Giles, C.H., Smith, D., Huitson, A. (1974) A general treatment and classification of the solute adsorption isotherm. J. Colloid Interface Sci47, 755–765
Gruhn, G. (1974) Thermodynamik der Mischphasen, Deutscher Verlag für Grundstoffindustrie Leipzig
Hashimoto, Y., Tokura, K., Ozaki, K., Strachan, W.M.J. (1982) A comparison of water solubilities by the flask and micro-column methods. Chemosphere11, 991–1001
Holloway, P.J. (1982) The chemical constitution of plant cutins. In: The plant cuticle, pp. 45–84. Cutler, D.F., Alvin, K.L., Price, C.E., eds. Academic Press, London New York
Katz, Y., Diamond, J.M. (1974) Thermodynamic constants for nonelectrolyte partitioning between dimyristoyl lecithin and water. J. Membr. Biol.17, 101–120
Kipling, J.J. (1965) Adsorption from solutions of nonelectrolytes. Academic Press, London New York
Korenman, Y.I., Alymova, A.T., Kobeleva, N.S. (1981) Temperature variation of the degree of sorption of methyl- and dimethylphenols on a macroporous cation-exchange resin. Russ. J. Phys. Chem.55, 696–698
Korenman, Y.I., Nefedova, T.A., Taldykina, S.N. (1977) Extraction of nitrophenols from aqueous solutions at different temperatures. Russ. J. Phys. Chem.51, 730–732
Landolt-Börnstein (1962) Zahlenwerte und Funktionen aus Naturwissenschaften und Technik, 6th edn., vol. 2, part 7, Springer, Berlin
OECD (1981) Guidelines for testing of chemicals. OECD, Paris
Perrin, R.D., Armarego, W.L.F., Perrin, D.R. (1980) Purification of laboratory chemicals. 2nd edn. Pergamon, Oxford New York
Riederer, M., Schönherr, J. (1984) Accumulation and transport of (2,4-dichlorophenoxy)acetic acid in plant cuticles: I. Sorption in the cuticular membrane and its components. Ecotoxicol. Environ. Safety8, 236–247
Riederer, M., Schönherr, J. (1985) Accumulation and transport of (2,4-dichlorophenoxy)acetic acid in plant cuticles: II. Permeability of the cuticular membrane. Ecotoxicol. Environ. Safety9, 196–208
Riederer, M., Schönherr, J. (1986) Covalent binding of chlorophenoxyacetic acids to plant cuticles. Arch. Environ. Contam. Toxicol.15, 97–105
Rogers, J.A., Wong, A. (1980) The temperature dependence and thermodynamics of partitioning of phenols in then-octanol-water system. Int. J. Pharmaceut.6, 339–348
Sachs, L. (1974) Angewandte Statistik. 4th edn. Springer, Berlin Heidelberg New York
Schmidt, H.W., Schönherr, J. (1982) Development of plant cuticles: occurrence and role of non-ester bonds in cutin ofClivia miniata Reg. leaves. Planta156, 380–384
Schönherr, J. (1982) Resistance of plant surfaces to water loss: transport properties of cutin, suberin and associated lipids. In: Encyclopedia of plant physiology, N.S., vol. 12 B, pp. 153–179, Pirson, A., Zimmermann, M.H., eds.. Springer, Berlin Heidelberg New York
Shafer, W.E., Schönherr, J. (1985) Accumulation and transport of phenol, 2-nitrophenol, and 4-nitrophenol in plant cuticles. Ecotoxicol. Environ. Safety10, 239–252
Sitte, P., Rennier, R. (1963) Untersuchungen an cuticularen Zellwandschichten. Planta60, 19–40
Sokal, R.R., Rohlf, F.J. (1981) Biometry, 2nd edn. Freeman, San Francisco
Urano, K., Koichi, Y., Nakazawa, Y. (1981) Equilibria for adsorption of organic compounds on activated carbons in aqueous solutions. J. Colloid Interface Sci.81, 477–485
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Riederer, M., Schönherr, J. Thermodynamic analysis of nonelectrolyte sorption in plant cuticles: The effects of concentration and temperature on sorption of 4-nitrophenol. Planta 169, 69–80 (1986). https://doi.org/10.1007/BF01369777
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01369777