Abstract
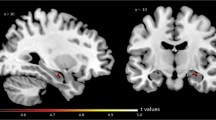
Iodine-123 labelled iomazenil (IMZ) is a specific tracer for the GABAA receptor, the dominant inhibitory synapse of the brain. The cerebral distribution volume (V d ) of IMZ may be taken as a quantitative measure of these synapses in Alzheimer's disease (AD), where synaptic loss tends indiscriminately to affect all cortical neurons, albeit more so in some areas than in others. In this pilot study we measured V d in six patients with probable AD and in five age-matched controls using a brain-dedicated single-photon emission tomography scanner allowing all cortical levels to be sampled simultaneously. Reduced values were found in all regions except in the occipital (visual) cortex. In particular, temporal and parietal cortexV d was significantly (P<0.02) reduced: temporalV d averaged 69 ml/ml in normals and 51 ml/ml in AD, and parietalV d averaged 71 ml/ml in normals and 48 ml/ml in AD. These results accord well with emission tomographic studies of blood flow or labelled glucose. This supports the idea that while only measuring a subpopulation of synapses, the IMZ method reflects synaptic loss and hence functional loss in AD. The method constitutes an in vivo version of synaptic quantitation that in histopathological studies has been shown to correlate closely with the mental deterioration in AD.
Similar content being viewed by others
References
Nordberg A. Neuroreceptor changes in Alzheimer disease.Cerebrovasc Brain Metab Rev 1992; 4: 303–328.
Greenamyre JT, Maragos F. Neurotransmitter receptors in Alzheimer disease.Cerebrovasc Brain Metab Rev 1993; 5: 61–94.
Oslen RW. The GABA postsynaptic membrane receptor ionophore complex.Mol Cell Biochem 1981; 39: 261–279.
Hunkeler W, Möhler H, Pieri L. Selective antagonist of benzodiazepines.Nature 1981; 290: 514–516.
Shinotoh H, Yamasaki T, Inoue O, Itch T, Suzuki K, Hashimoto K, Tateno Y, Ikeria H. Visualization of specific binding sites of benzodiazepine in human brain.J Nucl Med 1986; 27: 1593–1599.
Beer HF, Blauentein PA, Hasler PH, Delaloye B, Riccabona G, Bangerl I, Hunkeler W, Bonetti EP, Pieri L, Richards JG, Schubiger PA. In vivo and in vitro evaluation of iodine 123-RO16-0154: a new imaging agent for SPECT investigations of benzodiazepine receptors.J Nucl Med 1990; 31: 1007–1014.
Videboek C, Friberg L, Holm S, Wammen S, Foged C, Andersen JV, Dalgaard L, Lassen NA. Benzodiazepine receptor equilibrium constants for flumazenil and medazoiam determined in humans with the single photon emission computer tomography trace (123-I)iomazenil.Eur J Pharmacol 1993; 249: 43–51.
Abi-Dargham A, Laruelle M, Seibyl J, Rattner Z, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Bremmer JD, Hyde TM, Charney DS, Hoffer PB, Innis RB. SPECT measurement of benzodiazepine receptors in human brain with iodine-123-iomazenil: kinetic and equilibrium paradigms.J Nucl Med 1994; 35: 228–238.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease report of the NINCDS-ADRDA work group.Neurology 1984; 34: 939–944.
Laurell M, van Dyck C, Abi-Dargham A, Zea-Ponche Y, Zoghbai S, Charney D, Baldwin R, Hoffer P, Kung H, Innies R. Compartmental modeling of iodine-123 iodo-benzofuran binding to dopamine D2 receptors in healthy subjects.J Nucl Med 1994; 35: 743–754.
Onishi Y, Yonekura Y, Mukai T, Nishizawa S, Tanaka F, Okazawa H, Ishizu K, Fujita T, Hiroshi S, Konishi J. Simple quantification of benzodiazepine receptor binding and ligand transport using iodine-123-iomazenil and two SPECT scans.J Nucl Med 1995; 36: 1201–1210.
Iida H, Rob H, Bloomfield P, Masahiro M, Shuichi H, Matsutaro M, Atsushi I, Stefan E, Yasuo A, Iwao K, Kazuo U. A method to quantitate cerebral blood flow using a rotating gamma camera and iodine-123 iodamphetamine with one blood sample.Eur J Nucl Med 1994; 21: 1072–1084.
Odano I, Takahashi N, Ohkubo M, Yonekura Y SPECT measurement of benzodiazepine receptors in patients with Parkinson's disease with iodine-123 labeled iomazenil.J Nucl Med 1995;36:241P.
De Kosky ST, Bass NH. Biochemistry of senile dementia. In: Lajtha A, ed.Handbook of neurochemistry. New York: Plenum Press; 1985: 617–649
Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease.J Neurol Sci 1987; 78: 151–164.
Schell SW, De Kosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease.Neurobiol Aging 1990; 11: 29–37.
Masliah E, Terry RD, De Teresa RM, Hansen LA. Immunohistochemical quantification of the synapse related protein synaptophysin in Alzheimer's disease.Neurosci Lett 1989; 103: 234–239.
Hamos JE, De Gennaro LJ, Drachman DA. Synaptic loss in Alzheimer's disease and other dementias.Neurology 1989; 39: 355–361.
Terry RD, Masliah E, Salmon DP, Butters N, De Teresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment.Ann Neurol 1991; 30: 572–580.
Kanno I, Lassen NA. Two methods for calculation of regional cerebral blood flow from emission computed tomography of inert gas concentrations.J Comput Assist Tomogr 1979; 3: 71–76.
Rapoport SI. Positron emission tomography in Alzheimer's disease in relation to pathogenesis: a critical review.Cerebrovasc Brain Metab Rev 1991; 3: 297–335.
Waldemar G. Functional brain imaging with SPECT in normal aging and dementia.Cerebrovasc Brain Metab Rev 1995; 7: 89–130.
Weinberger DR, Jones D, Reba RC, Mann U, Coppola R, Gibson R, Gorey J, Braun A, Chase TN. A comparison of FDG PET and IQNB SPECT in normal subjects and in patients with dementia.J Neuropyschiatry Clin Neurosci 1992; 4: 239–248.
Wyper DJ, Brown D, Patterson J, Owens J, Hunter R, Teasdale E, McCulloch J. Deficits in iodine-labelled 3-quinuclidinyl benzilate binding in relation to cerebral blood flow in patients with Alzheimer's disease.Eur J Nucl Med 1993; 20: 379–386.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Soricelli, A., Postiglione, A., Grivet-Fojaja, M.R. et al. Reduced cortical distribution volume of iodine-123 iomazenil in Alzheimer's disease as a measure of loss of synapses. Eur J Nucl Med 23, 1323–1328 (1996). https://doi.org/10.1007/BF01367587
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01367587