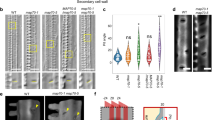
Summary
We have investigated the effects of microtubule stabilizing conditions upon microtubule patterns in protoplasts and developed a new method for producing protoplasts which have non-random cortical microtubule arrays. Segments of elongating pea epicotyl tissue were treated with the microtubule stabilizing drug taxol for 1 h before enzymatic digestion of the cell walls in the presence of the drug. Anti-tubulin immunofluorescence showed that 40 μM taxol preserved regions of ordered microtubules. The microtubules in these regions were arranged in parallel arrays, although the arrays did not always show the transverse orientation seen in the intact tissue. Protoplasts prepared without taxol had microtubules which were random in distribution. Addition of taxol to protoplasts with random microtubule arrangements did not result in organized microtubule arrays. Taxol-treated protoplasts were used to determine whether or not organized microtubule arrays would affect the organization of cell wall microfibrils as new walls were regenerated. We found that protoplasts from taxol-treated tissue which were allowed to regenerate cell walls produced organized arrays of microfibrils whose patterns matched those of the underlying microtubules. Protoplasts from untreated tissue synthesized microfibrils which were disordered. The synthesis of organized microfibrils by protoplasts with ordered microtubules arrays shows that microtubule arrangements in protoplasts influence the arrangement of newly synthesized microfibrils.
Similar content being viewed by others
Abbreviations
- DIC:
-
differential interference contrast
- DMSO:
-
dimethyl sulfoxide
- FITC:
-
fluorescein isothiocyanate
- IgG:
-
immunoglobulin G
- PIPES:
-
piperazine-N,N′-bis[2-ethane-sulfonic acid]
- PBS:
-
phosphate buffered saline
References
Brown RM (1985) Cellulose microfibril assembly and orientations: recent developments. J Cell Sci [Suppl 2]: 13–32
Burgess J (1983) Wall regeneration around isolated protoplasts. Int Rev Cytol [Suppl 16]: 55–78
Falconer MM, Seagull RW (1985) Xylogenesis in tissue culture: taxol effects on microtubule reorientation and lateral association in differentiating cells. Protoplasma 128: 157–166
Fischer JMC, Peterson CA, Bols NC (1985) A new fluorescent test for cell vitality using calcofluor white M 2 R. Stain Technol 60: 69–79
Galway ME, Hardham AR (1986) Microtubule reorganization, cell wall synthesis and establishment of the axis of elongation in regenerating protoplasts of the algaMougeotia. Protoplasma 135: 130–143
Giddings TH, Staehelin LA (1988) Spatial relationship between microtubules and plasma-membrane rosettes during the deposition of primary wall microfibrils inClosterium sp. Planta 173: 22–30
Hardham AR, Gunning BES (1978) Structure of cortical microtubule arrays in plant cells. J Cell Biol 77: 14–34
Hasezawa S, Hogetsu T, Syono K (1988) Rearrangement of cortical microtubules in elongating cells derived from tobacco protoplasts-a time course observation by immunofluorescence microscopy. J Plant Physiol 133: 46–51
Hayano S, Itoh T, Brown RM (1988) Orientation of microtubules during regeneration of cell wall in selected giant marine algae. Plant Cell Physiol 29: 785–793
Heath IB (1974) A unified hypothesis for the role of membrane bound enzyme complexes and microtubules in plant cell wall synthesis. J Theor Biol 48: 445–449
—, Seagull RW (1982) Oriented cellulose fibrils and the cytoskeleton: a critical comparison of models. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 163–182
Iwata K, Hogetsu T (1988) Arrangement of cortical microtubules inAvena coleoptiles and mesocotyls andPisum epicotyls. Plant Cell Physiol 29: 807–815
Lancelle SA, Callaham DA, Hepler PK (1986) A method for rapid freeze fixation of plant cells. Protoplasma 131: 153–165
Lloyd CW, Barlow PW (1982) The co-ordination of cell division and elongation: the role of the cytoskeleton. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 203–228
—, Slabas AR, Powell AJ, Lowe SB (1980) Microtubules, protoplasts and plant cell shape. An immunofluorescent study. Planta 147: 500–506
Maeda H, Ishida N (1967) Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J Biochem 62: 276–278
Marchant HJ (1979) Microtubules, cell wall deposition and the determination of plant cell shape. Nature 278: 167–168
— (1982) The establishment and maintenance of plant cell shape by microtubules. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 295–320
Osborn M, Weber K (1982) Immunofluorescence and immunocy-tochemical procedures with affinity purified antibodies: tubulin containing structures. Methods Cell Biol 24: 97–132
Pickett-Heaps JD (1967) The effects of colchicine on the ultrastructure of dividing cells, xylem wall differentiation and distribution of cytoplasmic microtubules. Dev Biol 15: 206–236
Quader H (1986) Cellulose microfibril orientation inOocystis solitaria: proof that microtubules control the alignment of the terminal complexes. J Cell Sci 83: 223–234
—, Herth W, Ryser U, Schnepf E (1987) Cytoskeletal elements in cotton seed hair development in vitro: their possible role in cell wall organization. Protoplasma 137: 56–62
Reinert J, Yeoman MM (1982) Plant cell and tissue culture-a laboratory manual. Springer, Berlin Heidelberg New York
Robinson DG, Quader H (1982) The microtubule-microfibril syndrome. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 109–126
Roland JC, Reis D, Vian B, Satiat-Jeunemaitre B, Mosiniak M (1987) Morphogenesis of plant cell walls at the supramolecular level: internal geometry and versatility of helicoidal expression. Protoplasma 140: 75–91
Schiff PB, Horwitz SB (1980) Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA 77: 1561–1565
Trass JA, Braat P, Emons AMC, Meekes H, Derksen J (1985) Microtubules in root hairs. J Cell Sci 76: 303–320
Van der Valk P, Rennie PJ, Connolly JA, Fowke LC (1980) Distribution of cortical microtubules in tobacco protoplasts. An immunofluorescence microscopic and ultrastructural study. Protoplasma 105: 27–43
Weerdenberg C, Seagull RW (1988) The effects of taxol and colchicine on microtubule and microfibril arrays in elongating plant cells in culture. Can J Bot 66: 1707–1716
—, Falconer MM, Setterfield G, Seagull RW (1986) Effects of taxol on microtubule arrays in cultured higher plant cells. Cell Motil Cytoskel 6: 469–478
Wick SM, Seagull RW, Osborn M, Weber K, Gunning BES (1981) Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol 89: 685–690
Williamson FA, Fowke LC, Weber G, Constabel F, Gamborg O (1977) Microfibril deposition on cultured protoplasts ofVicia hajastana. Protoplasma 91: 213–219
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Melan, M.A. Taxol maintains organized microtubule patterns in protoplasts which lead to the resynthesis of organized cell wall microfibrils. Protoplasma 153, 169–177 (1990). https://doi.org/10.1007/BF01354001
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01354001