Summary
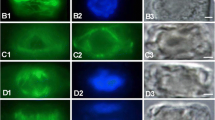
Differentiated mesophyll cells ofTriticum aestivum (cv. Star) exhibit a lobed outline resembling tube-shaped balloons with almost regularly spaced constrictions. It was shown that these constrictions are probably the result of hoops of wall reinforcements laid down during early stages of cell expansion. It appears that these hoops prevent expansion in the corresponding regions and thus give rise to the peculiar cell shape. The comparatively thin cell walls of the bulges are uniformly reinforced after the lobed shape is established.
By using immunofluorescence techniques a change in the pattern of cortical microtubule arrangement was observed which corresponded to the pattern of cell wall deposition. Discrete bands of microtubules were found beneath the sites of hoop reinforcement. These bands disintegrated during late stages of cell expansion with microtubules fanning out into the almost empty regions of the bulges.
Similar content being viewed by others
Abbreviations
- DMSO:
-
dimethyl sulfoxid
- EGTA:
-
ethylene glycol bis-(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid
- FITC:
-
fluorescein isothiocyanat
- MSB:
-
microtubule stabilizing buffer
- PBS:
-
phosphate buffered saline
- PIPES:
-
1,4-piperazine diethanesulfonic acid
- PMSF:
-
phenylmethyl sulfonylfluoride
References
Falconer MM, Seagull RW (1985) Xylogenesis in tissue culture: taxol effects on microtubule reorientation and lateral association in differentiating cells. Protoplasma 128: 157–166
Gerstenberger P, Leins P (1978) Rasterelektronenmikroskopische Untersuchungen an Blütenknospen vonPhysalis philadelphica (Solanaceae). Anwendung einer neuen Präparationsmethode. Ber Deutsch Bot Ges 91: 381–387
Gunning BES, Hardham AR (1982) Microtubules. Ann Rev Plant Physiol 33: 651–698
Hardham AR, Gunning BES (1979) Interpolation of microtubules into cortical arrays during cell elongation and differentiation in roots ofAzolla pinnata. J Cell Sci 37: 411–442
Hepler PK, Fosket DE (1971) The role of microtubules in vessel member differentiation inColeus. Protoplasma 72: 213–236
—, Palevitz BA (1974) Microtubules and microfilaments. Ann Rev Plant Physiol 25: 309–362
Iwata K, Hogetsu T (1988) Arrangement of cortical microtubules inAvena coleoptiles and mesocotyls andPisum epicotyls. Plant Cell Physiol 29: 807–815
Kobayashi H, Fukuda H, Shibaoka H (1988) Interrelation between the spatial disposition of actin filaments and microtubules during the differentiation of tracheary elements in culturedZinnia cells. Protoplasma 143: 29–37
Lloyd CW (1987) The plant cytoskeleton: the impact of fluorescence microscopy. Ann Rev Plant Physiol 38: 119–139
—, Wells B (1985) Microtubules are at the tips of root hairs and form helical patterns corresponding to inner wall fibrils. J Cell Sci 75: 225–238
Mayer RJ, Walker JH (1987) Immunochemical methods in cell and molecular biology. Academic Press, London San Diego
Palevitz BA, Hepler PK (1976) Cellulose microfibril orientation and cell shaping in developing guard cells ofAllium: the role of microtubules and ion accumulation. Planta 132: 71–93
Preston RD (1988) Cellulose-microfibril-orienting mechanisms in plant cell walls. Planta 174: 67–74
Quader H, Deichgräber G, Schnepf E (1986) The cytoskeleton ofCobaea seed hairs: patterning during cell-wall differentiation. Planta 168: 1–10
Seagull RW (1989) The plant cytoskeleton. CRC Crit Rev Plant Sci 8: 131–167
Schneider B, Herth W (1986) Distribution of plasma membrane rosettes and kinetics of cellulose formation in xylem development of higher plants. Protoplasma 131: 142–152
Schnepf E (1973) Microtubulus-Anordnung und -Umordnung, Wandbildung und Zellmorphogenese in jungenSphagnum-Blättchen. Protoplasma 78: 145–173
Sinnott EW, Bloch R (1945) The cytoplasmic basis of intercellular patterns in vascular differentiation. Am J Bot 32: 151–156
Valk HC van der, Blaas J, Eck JW van, Verhoeven HA (1988) Vital DNA staining of agarose-embedded protoplasts and cell suspensions ofNicotiana plumbaginifolia. Plant Cell Rep 7: 489–492
Wernicke W, Milkovits L (1984) Developmental gradients in wheat leaves-response of leaf segements in different genotypes cultured in vitro. J Plant Physiol 115: 49–58
Wick SM, Duniec J (1983) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I. Preprophase band development and concomitant appearance of nuclear envelope-associated tubulin. J Cell Biol 97: 235–243
—, Seagull RW, Osborn M, Weber K, Gunning BES (1981) Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol 89: 685–690
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jung, G., Wernicke, W. Cell shaping and microtubules in developing mesophyll of wheat (Triticum aestivum L.). Protoplasma 153, 141–148 (1990). https://doi.org/10.1007/BF01353998
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01353998