Summary
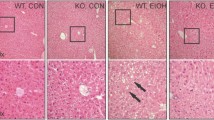
Alcohol was administered chronically to female Sprague Dawley rats in a nutritionally adequate totally liquid diet for 28 days. This resulted in hepatic steatosis and lipid peroxidation. Taurine, when co-administered with alcohol, reduced the hepatic steatosis and completely prevented lipid peroxidation. The protective properties of taurine in preventing fatty liver were also demonstrated histologically. Although alcohol was found not to affect the urinary excretion of taurine (a non-invasive marker of liver damage), levels of serum and liver taurine were markedly raised in animals receiving alcohol + taurine compared to animals given taurine alone. The ethanol-inducible form of cytochrome P-450 (CYP2E1) was significantly induced by alcohol; the activity was significantly lower than controls and barely detectable in animals fed the liquid alcohol diet containing taurine. In addition, alcohol significantly increased homocysteine excretion into urine throughout the 28 day period of ethanol administration; however, taurine did not prevent this increase. There was evidence of slight cholestasis in animals treated with alcohol and alcohol + taurine, as indicated by raised serum bile acids and alkaline phosphatase (ALP). The protective effects of taurine were attributed to the potential of bile acids, especially taurine conjugated bile acids (taurocholic acid) to inhibit the activity of some microsomal enzymes (CYP2E1). Thesein vivo findings demonstrate for the first time that hepatic steatosis and lipid peroxidation, occurring as a result of chronic alcohol consumption, can be ameliorated by administration of taurine to rats.
Similar content being viewed by others
References
Albano E, Tomasi E, Persson J-O, Terelius Y, Goria-Gatti L, Ingelman-Sundberg M, Dianzani MU (1991) Role of ethanol-inducible cytochrome P-450 (P-4502E1) in catalyzing the free radical activation of aliphatic alcohols. Biochem Pharm 41: 1895–1902
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Butler WM, Maling HM, Horning HG, Brodie BB (1962) The direct determination of liver triglycerides. J Lipid Res 2: 95–96
Chen J, Farrell GC (1996) Bile acids produce a generalized reduction of the catalytic activity of cytochromes P-450 and other hepatic microsomal enzymes in vitro: relevance to drug metabolism in experimental cholestasis. J Gastroenterol Hepatol 11: 870–877
Chen J, Murray M, Liddle C, Jiang XM, Farrell GC (1995) Downregulation of malespecific cytochrome P-450s 2C11 and 3A2 in bile duct-ligated male rats: importance to reduced hepatic content of cytochrome P-450 in cholestasis. Hepatology 22: 580–587
De Master EG, Redfern B (1987) High performance liquid chromatography of hepatic thiols with electrochemical detection. In: Jakoby WB, Griffith OW (eds) Methods of enzymology, vol 193. Academic Press, New York, pp 110
Ekstrom G, Ingelman-Sundberg M (1989) Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-4502E1). Biochem Pharm 38: 1313–1319
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82: 70–77
Farinati F, Lieber CS, Garro AJ (1989) Effects of chronic ethanol consumption on carcinogen activating and detoxifying systems in rat upper alimentary tract tissue. Alcohol Clin Exp Res 13: 357–360
Fernández-Checa JC, Hirano T, Tsukamoto H, Kaplowitz N (1993) Mitochondrial glutathione depletion in alcoholic liver disease. Alcohol 10: 469–475
Fortin L-J, Genest J (1995) Measurement of homocysteine in the prediction of arteriosclerosis. Clin Biochem 28: 155–162
Fukaya Y, Senda N, Fujita A, Imai S, Sawada I (1996) Combined effect of taurine and ox bile on biliary flow. Adv Exp Med Biol 403: 93–97
Griffith OW (1980) Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106: 207–212
Harrison DJ, Burt AD (1993) Pathology of alcoholic liver disease. Bailliere's Clinical Gastroenterol 7: 641–662
Horning MG, Wakabayashi M, Maling HM (1963) Biochemical processes involved in the synthesis, accumulation and release of triglycerides by the liver. In: Horning EC (ed) Mode of action of drugs. Effects of drugs on synthesis and mobilization of lipids, vol 2. Pergamon, Oxford, p 13
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72: 101–163
Jenner AM, Timbrell JA (1994) Effect of acute and repeated exposure to low doses of hydrazine on hepatic microsomal enzymes and biochemical parameters in vivo. Arch Toxicol 68: 240–245
Kawase T, Kato S, Lieber CS (1989) Lipid peroxidation and antioxidant defense systems in rat liver after chronic ethanol feeding. Hepatology 10: 815–821
Kawata S, Imai Y, Inada M, Tamura S, Miyoshi S, Nishikawa M, Minami Y, Tarui S (1987) Selective reduction of hepatic cytochrome P-450 content in patients with intrahepatic cholestasis. A mechanism for impairment of microsomal drug oxidation. Gastroenterol 92: 299–303
Kenyon SH, Nicolaou A, Gibbons WA (1998) The effect of ethanol and its metabolites upon methionine synthase activity in vitro. Alcohol 15: 305–309
Lake BG (1987) Investigations and characterization of microsomal fractions for studies of xenobiotic metabolism. In: Snell K, Mullock B (eds) Biochemical toxicology: a practical approach. IRL Press, Oxford, pp 183–215
Lieber CS (1993) Biochemical factors in alcoholic liver disease. Semin Liver Dis 13: 136–153
Lieber CS (1997a) Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev 77: 517–544
Lieber CS (1997b) Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol 38: 601–628
Lieber CS, DeCarli LM (1989) Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211
Lieber CS, Savolainen M (1984) State of the art. Ethanol and lipids. Alcoholism Clin Exp Res 8: 409–423
Lieber CS, Casini A, DeCarli LM, Kim C, Lowe N, Sasaki R, Leo MA (1990) S-adenosylL-methionine attenuates alcohol-induced liver injury in the baboon. Hepatology 11: 165–172
Lieber CS, Robins SJ, Li J., DeCarli LM, Mak KM, Faulo JM, Leo MA (1994) Phosphatidylcholine protects against fibrosis and cirrhosis in the baboon. Gastroenterol 106: 152–159
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 263: 265–275
McCloskey LP, Mahaney P (1981) An enzymatic assay for acetaldehyde in grape juice and wine. Am J Enol Vitic 32: 159–162
Morimoto M, Hagbövrk A-L, Nanji AA, Ingelman-Sundberg M, Lindros KO, Fu PC, Albano E, French SW (1993) Role of cytochrome P-4502E1 in alcoholic liver disease pathogenesis. Alcohol 10: 459–464
Müller A, Sies H (1982) Role of alcohol dehydrogenase activity and of acetaldehyde in ethanol-induced ethane and pentane production by isolated perfused rat liver. Biochem J 206: 153–156
Nakashima T, Takino T, Kuriyama K (1983) Therapeutic and prophylactic effects of taurine administration on experimental liver injury. In: Kuriyama K, Huxtable RJ, Iwata H (eds) Sulphur amino acids: biochemical and clinical aspects. Alan R Liss Inc., New York, pp 449–459
Nicolaou A, Waterfield CJ, Kenyon SH, Gibbons WA (1997) The inactivation of methionine synthase in isolated rat hepatocytes by sodium nitroprusside. Eur J Biochem 244: 876–882
Omura T, Sato R (1964) The carbon monoxide binding pigment of liver microsomes. Evidence of its haemoprotein value. J Biol Chem 239: 2370–2378
Pietrzak ER, Shanley BC, Kroon PA (1995) Antibodies made against a formaldehydeprotein adduct cross react with an acetaldehyde-protein adduct. Implications for the origin of antibodies in human serum which recognize acetaldehyde-protein adducts. Alcohol Alcohol 30: 373–378
Prough RA, Burke MD, Mayer RT (1978) In: Fleischer S, Packer L (eds) Methods in enzymology, vol 52. Academic Press, New York, pp 372–377
Reinke LA, Lai EK, DuBose CM, McCay PB (1987) Reactive free radical generation in vivo in heart and liver of ethanol-fed rats: correlation with radical formation in vitro. Proc Natl Acad Sci USA 84: 9223–9227
Sawicki E, Stanley TW, Johnson H (1963) Comparison of spectrophotometric and spectrophotofluorometric methods for the determination of malonaldehyde. Anal Chem 35: 199–205
Schapiro RH, Scheig RL, Drummey GD, Mendelson JH, Isselbacher KJ (1965) Effect of prolonged ethanol ingestion on the transport and metabolism of lipids in man. N Engl J Med 272: 610
Seabra V, Timbrell JA (1997) Modulation of taurine levels in the rat liver alters methylene dianiline hepatotoxicity. Toxicology 122: 193–204
Shaw S, Jayatilleke E, Ross WA (1981) Ethanol-induced lipid peroxidation: potentiation by long-term alcohol feeding and attenuation by methionine. J Lab Clin Med 98: 417–424
Shaw S, Jayatilleke E, Lieber CS (1988) Lipid peroxidation as a mechanism of alcoholic liver injury: role of iron mobilization and microsomal induction. Alcohol 5: 135–140
Timbrell JA, Seabra V, Waterfield CJ (1995) The in vivo and in vitro protective properties of taurine. Gen Pharmac 26: 453–462
Trimble KC, Molloy AM, Scott JM, Weir DG (1993) The effect of ethanol on one-carbon metabolism: increased methionine catabolism and lipotrope methyl-group wastage. Hepatology 18: 984–989
Tsuboi N, Yoshida H, Shibamura K, Hikita M, Tomonari H, Kuriyama S, Sakai O (1997) Acute renal failure after binge drinking of alcohol and nonsteroidal anti-inflammatory drug ingestion. Intern Med 36: 102–106
Vendemiale G, Lieber CS (1984) Acute and chronic effects of ethanol on biliary secretion of bilirubin and bile acids. Subst Alcohol Actions Misuse 5: 307–317
Vessey DA (1978) The biochemical basis for the conjugation of bile acids with either glycine or taurine. Biochem J 174: 621–626
Watanabe A, Hobara N, Nagashima H (1985) Lowering of liver acetaldehyde but not ethanol concentrations by pretreatment with taurine in ethanol-loaded rats. Experientia 41: 1421–1422
Waterfield CJ (1994) Determination of taurine in biological samples and isolated hepatocytes by high performance liquid chromatography with fluorimetric detection. J Chromatography 657: 37–45
Waterfield CJ, Turton JA, Scales MDC, Timbrell JA (1993a) Reduction of liver taurine in rats by β-alanine treatment increases carbon tetrachloride toxicity. Toxicology 77: 7–20
Waterfield CJ, Turton JA, Scales MDC, Timbrell JA (1993b) Investigations into the effects of various hepatotoxin compounds on urinary and liver taurine levels in rats. Arch Toxicol 67: 244–254
Waterfield CJ, Asker DA, Timbrell JA (1996) Does urinary taurine reflect changes in protein metabolism? A study with cycloheximide in rats. Biomarkers 1: 107–114
Yan CC, Bravo E, Cantafora A (1993) Effect of taurine levels on liver lipid metabolism: an in vivo study in the rat. Proc Soc Exp Biol Med 202: 88–96
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kerai, M.D.J., Waterfield, C.J., Kenyon, S.H. et al. Taurine: Protective properties against ethanol-induced hepatic steatosis and lipid peroxidation during chronic ethanol consumption in rats. Amino Acids 15, 53–76 (1998). https://doi.org/10.1007/BF01345280
Issue Date:
DOI: https://doi.org/10.1007/BF01345280